Western blotting is a powerful analytical technique used to detect specific proteins in a complex mixture, employing gel electrophoresis followed by transfer to a membrane for antibody probing. This method provides precise information about protein size and abundance, playing a crucial role in research areas like molecular biology and diagnostics. Explore the rest of the article to deepen your understanding of the Western blotting process and its applications.
Table of Comparison
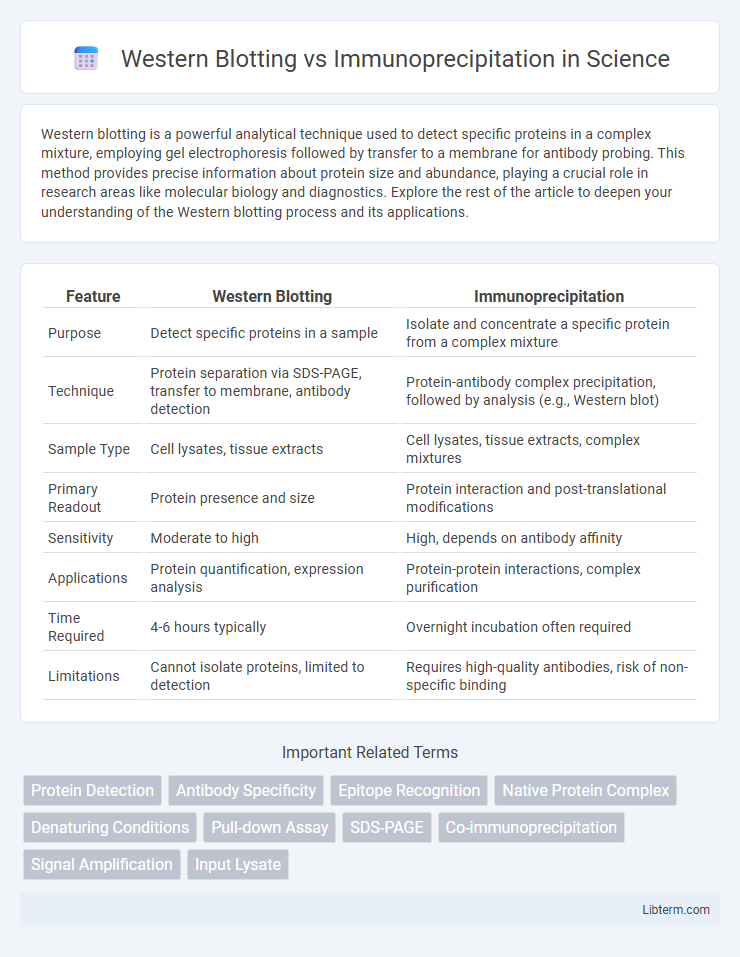
| Feature | Western Blotting | Immunoprecipitation |
|---|---|---|
| Purpose | Detect specific proteins in a sample | Isolate and concentrate a specific protein from a complex mixture |
| Technique | Protein separation via SDS-PAGE, transfer to membrane, antibody detection | Protein-antibody complex precipitation, followed by analysis (e.g., Western blot) |
| Sample Type | Cell lysates, tissue extracts | Cell lysates, tissue extracts, complex mixtures |
| Primary Readout | Protein presence and size | Protein interaction and post-translational modifications |
| Sensitivity | Moderate to high | High, depends on antibody affinity |
| Applications | Protein quantification, expression analysis | Protein-protein interactions, complex purification |
| Time Required | 4-6 hours typically | Overnight incubation often required |
| Limitations | Cannot isolate proteins, limited to detection | Requires high-quality antibodies, risk of non-specific binding |
Introduction to Protein Detection Methods
Western blotting enables the detection and quantification of specific proteins by separating them via gel electrophoresis followed by antibody-based identification, providing molecular weight and expression data. Immunoprecipitation isolates proteins from complex mixtures using antigen-specific antibodies, facilitating the study of protein-protein interactions and post-translational modifications. Both techniques are essential for analyzing protein presence, abundance, and functional relationships in biological samples.
What is Western Blotting?
Western blotting is a highly sensitive analytical technique used to detect specific proteins in a complex sample through antibody binding, enabling the identification and quantification of target proteins. This method involves protein separation by gel electrophoresis, transfer onto a membrane, and incubation with labeled antibodies for visualization. Western blotting is widely used in molecular biology and biochemistry for protein expression analysis, post-translational modifications, and disease biomarker detection.
What is Immunoprecipitation?
Immunoprecipitation is a technique used to isolate a specific antigen from a complex mixture through the use of an antibody that binds to that target protein. The antigen-antibody complex is then precipitated out of solution, allowing for subsequent analysis of the purified protein, often by Western blotting. This method is essential for studying protein-protein interactions, post-translational modifications, and functional characterization of proteins within a cellular context.
Key Principles of Western Blotting
Western blotting is based on the separation of proteins by gel electrophoresis, followed by their transfer onto a membrane and detection using specific antibodies, enabling precise identification and quantification of target proteins. The technique relies on primary antibodies that bind to the protein of interest and secondary antibodies conjugated to enzymes or fluorescent markers for signal amplification. This method provides high specificity and sensitivity in analyzing protein expression levels and post-translational modifications.
Core Mechanisms of Immunoprecipitation
Immunoprecipitation (IP) isolates specific proteins from complex mixtures using antigen-antibody binding and subsequent capture by protein A/G beads, allowing targeted protein analysis. This core mechanism leverages antibody specificity to enrich low-abundance proteins prior to detection or further characterization. Compared to Western blotting, which separates proteins by gel electrophoresis before antibody detection, IP emphasizes selective protein enrichment through immune complex precipitation.
Applications in Molecular Biology Research
Western blotting is primarily used for detecting and quantifying specific proteins within complex mixtures, enabling analysis of protein expression, post-translational modifications, and molecular weight. Immunoprecipitation isolates specific proteins or protein complexes from cell lysates, facilitating studies on protein-protein interactions, signaling pathways, and identification of binding partners. Both techniques are complementary in molecular biology for elucidating protein function, regulation, and cellular mechanisms.
Strengths and Limitations: Western Blotting
Western blotting offers high specificity and sensitivity for detecting target proteins, providing quantitative data on protein size and expression levels. It requires relatively small sample amounts but may be limited by antibodies' quality and the inability to analyze protein interactions in native conditions. The technique is time-consuming and less effective for low-abundance proteins without prior enrichment steps.
Strengths and Limitations: Immunoprecipitation
Immunoprecipitation excels in isolating and enriching specific proteins or protein complexes from complex samples, enabling detailed studies of protein interactions and post-translational modifications. However, its limitations include potential nonspecific binding, dependence on high-quality antibodies, and relatively low throughput compared to methods like Western blotting. The technique also requires optimization of lysis conditions and antibody concentration to minimize background and increase specificity.
Western Blotting vs Immunoprecipitation: Key Differences
Western Blotting detects and quantifies specific proteins within a complex sample by separating proteins via gel electrophoresis and transferring them onto a membrane for antibody probing, providing information on protein size and expression levels. Immunoprecipitation isolates a target protein from a mixture using specific antibodies conjugated to beads, enabling the study of protein-protein interactions and post-translational modifications. The key differences lie in Western Blotting's focus on protein detection and quantification post-separation, whereas Immunoprecipitation emphasizes protein isolation and analysis of binding partners or protein complexes.
Choosing the Right Technique for Your Study
Western blotting enables the detection and quantification of specific proteins in complex samples through antibody binding and gel electrophoresis, ideal for assessing protein expression or modifications. Immunoprecipitation isolates proteins or protein complexes from a mixture using an antibody, facilitating studies of protein interactions, post-translational modifications, or purification for downstream analysis. Selecting between these techniques depends on study goals: Western blotting suits quantitative expression analysis, whereas immunoprecipitation is optimal for investigating protein-protein interactions and complex composition.
Western Blotting Infographic
 libterm.com
libterm.com