Microcrystalline structures consist of tiny, closely packed crystals that enhance the material's strength and durability. These formations are commonly found in metals, ceramics, and certain polymers, contributing to improved mechanical and optical properties. Explore the rest of the article to understand how microcrystalline materials impact your everyday products and industrial applications.
Table of Comparison
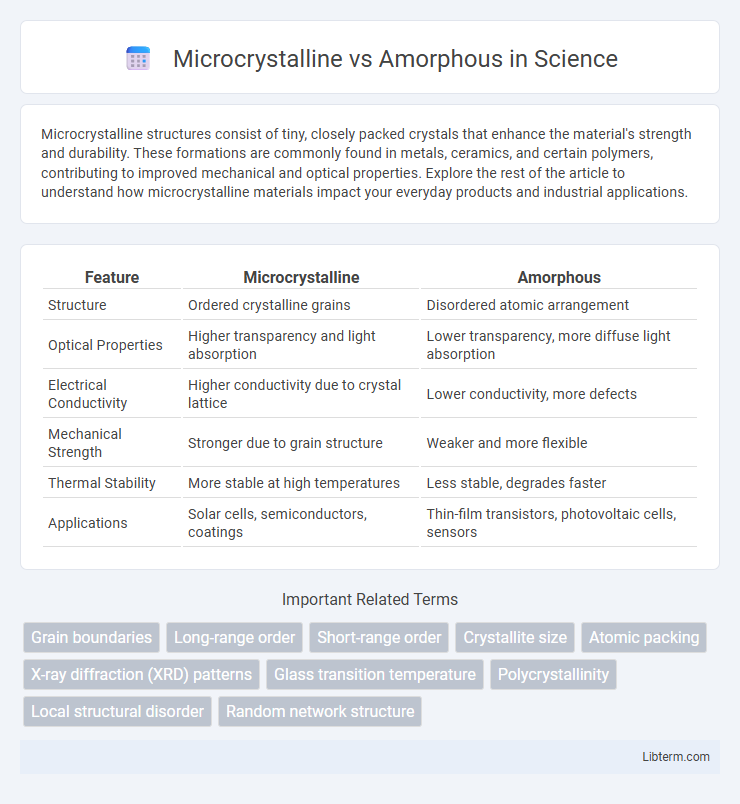
| Feature | Microcrystalline | Amorphous |
|---|---|---|
| Structure | Ordered crystalline grains | Disordered atomic arrangement |
| Optical Properties | Higher transparency and light absorption | Lower transparency, more diffuse light absorption |
| Electrical Conductivity | Higher conductivity due to crystal lattice | Lower conductivity, more defects |
| Mechanical Strength | Stronger due to grain structure | Weaker and more flexible |
| Thermal Stability | More stable at high temperatures | Less stable, degrades faster |
| Applications | Solar cells, semiconductors, coatings | Thin-film transistors, photovoltaic cells, sensors |
Introduction to Microcrystalline and Amorphous Materials
Microcrystalline materials consist of small, well-ordered crystals with grain sizes typically less than 1 micron, offering higher stability and distinct electrical properties compared to amorphous counterparts. Amorphous materials lack a long-range ordered crystal structure, resulting in isotropic properties and often enhanced flexibility in applications such as thin-film technology. Understanding the structural differences between microcrystalline and amorphous materials is crucial for optimizing their performance in solar cells, semiconductors, and electronic devices.
Structural Differences: Microcrystalline vs Amorphous
Microcrystalline materials consist of tightly packed crystals with long-range atomic order, whereas amorphous materials lack this ordered structure, exhibiting random atomic arrangements. The microcrystalline structure enhances mechanical strength and thermal conductivity due to its defined grain boundaries, in contrast to the isotropic and often less dense nature of amorphous solids. These structural differences significantly influence the physical properties and applications of each material type.
Key Properties of Microcrystalline Materials
Microcrystalline materials possess a well-defined crystalline structure with grain sizes typically less than 10 micrometers, resulting in higher mechanical strength and thermal stability compared to amorphous counterparts. These materials exhibit enhanced electrical conductivity and improved optical properties due to their ordered atomic arrangement. The key properties of microcrystalline substances include increased hardness, resistance to wear, and superior structural integrity, making them essential in semiconductor and photovoltaic applications.
Key Properties of Amorphous Materials
Amorphous materials lack a long-range ordered crystal structure, resulting in unique properties such as isotropy, where physical properties are uniform in all directions, and higher optical transparency compared to microcrystalline counterparts. These materials often exhibit enhanced chemical reactivity and faster diffusion rates due to their disordered atomic arrangement. Their low density of defects and absence of grain boundaries contribute to improved mechanical flexibility and uniform electronic properties critical in thin-film applications and advanced semiconductor devices.
Manufacturing Processes and Formation
Microcrystalline materials form through controlled crystallization processes involving slow cooling or annealing, allowing atoms to arrange into ordered, tightly packed crystal structures. Amorphous materials result from rapid cooling or quenching that prevents atom organization, yielding disordered, non-crystalline structures. Manufacturing techniques like chemical vapor deposition and sputtering can produce amorphous thin films, while techniques such as zone refining facilitate microcrystalline growth by promoting atomic alignment.
Applications in Industry and Technology
Microcrystalline materials, characterized by their ordered crystalline structures, are widely used in the solar photovoltaic industry for efficient thin-film solar cells due to their superior electronic properties and stability. Amorphous materials, lacking long-range order, find applications in flexible electronics, thin-film transistors, and low-cost solar panels where flexibility and light absorption are essential. Industrial technologies exploit the distinct electrical and mechanical properties of microcrystalline and amorphous materials to optimize performance in semiconductor devices, battery electrodes, and optical coatings.
Mechanical Strength and Durability Comparison
Microcrystalline materials exhibit higher mechanical strength due to their well-ordered atomic structure, which enhances resistance to deformation and fracture compared to amorphous materials. Amorphous solids lack a crystalline lattice, making them generally more brittle and less durable under mechanical stress. The densely packed grains in microcrystalline samples provide superior durability and resistance to wear, making them preferable for applications requiring long-term mechanical stability.
Thermal and Electrical Conductivity Differences
Microcrystalline materials exhibit higher thermal and electrical conductivity due to their ordered atomic structure, which facilitates efficient charge and heat transfer. In contrast, amorphous materials have disordered atomic arrangements that scatter electrons and phonons, resulting in lower conductivity levels. These differences make microcrystalline structures preferable for applications requiring rapid heat dissipation and electrical conduction, such as in photovoltaics and microelectronics.
Advantages and Limitations of Each Structure
Microcrystalline materials offer high stability and better mechanical strength due to their ordered grain structures, enhancing durability and conductivity in electronic applications; however, their synthesis can be complex and costly. Amorphous materials exhibit superior flexibility and uniformity, allowing for easier fabrication and adaptability in thin-film technologies, yet they generally suffer from lower electrical conductivity and less thermal stability. Choosing between microcrystalline and amorphous structures depends on the specific application requirements, balancing performance attributes like stability versus flexibility.
Choosing Between Microcrystalline and Amorphous: Factors to Consider
Choosing between microcrystalline and amorphous materials involves evaluating factors such as structural properties, durability, and application-specific requirements. Microcrystalline substances feature a well-ordered crystal lattice that enhances mechanical strength and resistance to wear, making them ideal for industrial and technological uses. In contrast, amorphous materials lack long-range order, offering flexibility and improved optical or magnetic characteristics suitable for specialized applications in electronics and coatings.
Microcrystalline Infographic
 libterm.com
libterm.com