Chemical reactions involve the transformation of substances through breaking and forming chemical bonds, resulting in new products with different properties. Understanding reaction mechanisms, types, and conditions allows you to predict outcomes and control processes in various fields, from industrial manufacturing to biological systems. Explore the rest of the article to deepen your grasp of how chemical reactions shape the world around you.
Table of Comparison
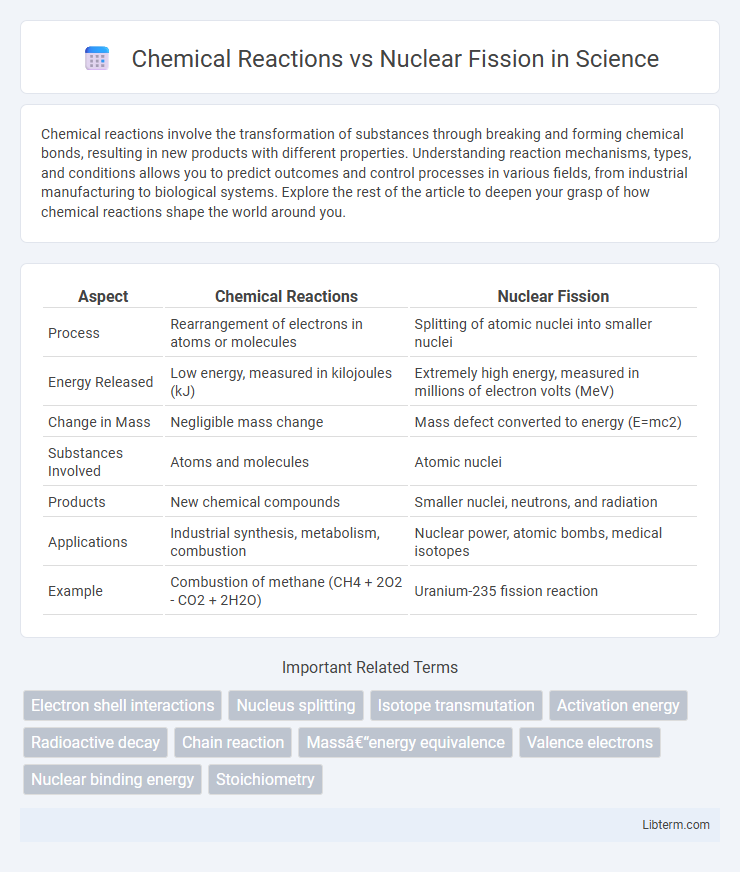
| Aspect | Chemical Reactions | Nuclear Fission |
|---|---|---|
| Process | Rearrangement of electrons in atoms or molecules | Splitting of atomic nuclei into smaller nuclei |
| Energy Released | Low energy, measured in kilojoules (kJ) | Extremely high energy, measured in millions of electron volts (MeV) |
| Change in Mass | Negligible mass change | Mass defect converted to energy (E=mc2) |
| Substances Involved | Atoms and molecules | Atomic nuclei |
| Products | New chemical compounds | Smaller nuclei, neutrons, and radiation |
| Applications | Industrial synthesis, metabolism, combustion | Nuclear power, atomic bombs, medical isotopes |
| Example | Combustion of methane (CH4 + 2O2 - CO2 + 2H2O) | Uranium-235 fission reaction |
Introduction to Chemical Reactions and Nuclear Fission
Chemical reactions involve the rearrangement of electrons between atoms, resulting in the formation of new substances without altering the nuclei of atoms. Nuclear fission, by contrast, is a process where an atomic nucleus splits into two or more smaller nuclei, releasing a significant amount of energy due to the conversion of mass into energy according to Einstein's equation E=mc2. While chemical reactions typically involve energy changes on the scale of electron volts, nuclear fission releases energy millions of times greater, making it a powerful source for nuclear power and weapons.
Fundamental Differences: Chemical vs Nuclear Processes
Chemical reactions involve the rearrangement of electrons in the outer shells of atoms, changing molecular structures without altering atomic nuclei, resulting in energy changes typically on the order of electron volts. Nuclear fission entails splitting an atomic nucleus into smaller nuclei, releasing vastly greater energy measured in millions of electron volts due to changes in nuclear binding energy. Unlike chemical processes, nuclear fission transforms elements by altering the number of protons and neutrons, significantly impacting atomic mass and nuclear identity.
How Chemical Reactions Work
Chemical reactions involve the rearrangement of electrons in the outer shells of atoms, forming or breaking chemical bonds without altering the nuclei of the atoms involved. These reactions release or absorb energy through the making and breaking of bonds, typically measured in kilojoules per mole. In contrast, nuclear fission splits atomic nuclei, releasing millions of times more energy than chemical reactions by converting mass into energy according to Einstein's equation, E=mc2.
The Science Behind Nuclear Fission
Nuclear fission is a process where the nucleus of a heavy atom, such as uranium-235 or plutonium-239, splits into smaller nuclei, releasing a significant amount of energy due to the conversion of mass into energy as described by Einstein's equation E=mc2. This reaction differs fundamentally from chemical reactions, which involve electron exchanges and rearrangements without altering atomic nuclei. The science behind nuclear fission involves neutron-induced splitting, chain reactions, and the release of neutrons that perpetuate the process, enabling applications in nuclear power generation and atomic weaponry.
Energy Output: Chemical Reactions vs Nuclear Fission
Nuclear fission releases energy millions of times greater than chemical reactions due to the conversion of a small amount of mass into energy, as described by Einstein's equation E=mc2. Chemical reactions involve the rearrangement of electrons in atoms, producing energy measured in electron volts, whereas nuclear fission breaks atomic nuclei, releasing energy on the order of millions of electron volts per event. This immense difference in energy output explains why nuclear fission powers reactors and weapons, while chemical reactions fuel everyday combustion and metabolism processes.
Everyday Examples of Chemical Reactions
Chemical reactions occur in everyday activities such as cooking, where heat causes proteins to denature and sugars to caramelize, and in respiration, where glucose reacts with oxygen to release energy. Combustion of fuels like gasoline in car engines is another common chemical reaction essential for transportation. These processes involve the rearrangement of electrons in atoms without changing the atomic nuclei, unlike nuclear fission, which splits atomic nuclei and releases far greater energy.
Real-World Applications of Nuclear Fission
Nuclear fission powers approximately 10% of the world's electricity through nuclear reactors, offering a low-carbon energy source that significantly reduces greenhouse gas emissions compared to fossil fuels. In medicine, nuclear fission produces isotopes like molybdenum-99, essential for diagnostic imaging and cancer treatment. Additionally, nuclear fission is integral to naval propulsion systems, enabling long-duration operations of aircraft carriers and submarines with high energy density and efficiency.
Safety and Environmental Impacts
Chemical reactions typically involve the rearrangement of electrons and release relatively low amounts of energy, resulting in minimal environmental hazards when properly managed. Nuclear fission generates significantly higher energy through the splitting of atomic nuclei, posing risks such as radioactive waste, potential radiation exposure, and long-term environmental contamination. Safety measures for nuclear fission require stringent containment systems and waste disposal protocols, whereas chemical reactions generally demand standard laboratory safety practices and environmental controls.
Role in Power Generation and Industry
Chemical reactions involve the rearrangement of electrons between atoms to form new substances, providing energy primarily through combustion processes used in industries and power plants for heat and electricity generation. Nuclear fission splits the nucleus of heavy atoms like uranium or plutonium, releasing significantly more energy per reaction, making it a critical technology in nuclear power plants for large-scale, low-carbon electricity production. The industrial application of nuclear fission extends to medical isotope production and naval propulsion, whereas chemical reactions dominate manufacturing, petrochemical processes, and everyday energy needs.
Future Perspectives: Advancements in Chemical and Nuclear Technologies
Future advancements in chemical technologies focus on catalysis and green chemistry to enhance reaction efficiency and sustainability, reducing environmental impact. Nuclear fission research prioritizes developing safer reactors, such as small modular reactors (SMRs), and advanced fuel cycles to improve energy output and waste management. Integration of digital monitoring and AI-driven control systems is expected to optimize both chemical processes and nuclear fission operations, driving innovation in energy production and material synthesis.
Chemical Reactions Infographic
 libterm.com
libterm.com