Saturated fats are types of dietary fats found in animal products and some plant oils, known for their solid state at room temperature. Excessive consumption of saturated fats can raise cholesterol levels, increasing the risk of heart disease. Discover how to balance your intake and make healthier choices by reading the full article.
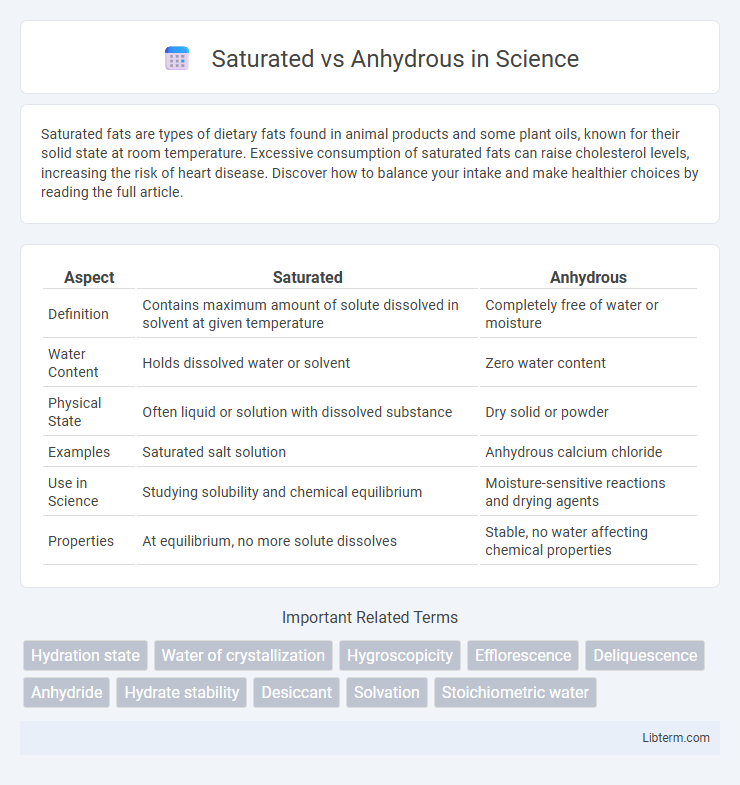
Table of Comparison
| Aspect | Saturated | Anhydrous |
|---|---|---|
| Definition | Contains maximum amount of solute dissolved in solvent at given temperature | Completely free of water or moisture |
| Water Content | Holds dissolved water or solvent | Zero water content |
| Physical State | Often liquid or solution with dissolved substance | Dry solid or powder |
| Examples | Saturated salt solution | Anhydrous calcium chloride |
| Use in Science | Studying solubility and chemical equilibrium | Moisture-sensitive reactions and drying agents |
| Properties | At equilibrium, no more solute dissolves | Stable, no water affecting chemical properties |
Understanding Saturated and Anhydrous Compounds
Saturated compounds contain the maximum number of hydrogen atoms attached to carbon atoms, resulting in single bonds only, which influences their stability and reactivity. Anhydrous compounds lack water molecules in their structure, often increasing their purity and altering physical properties compared to their hydrated counterparts. Understanding the differences between saturated and anhydrous compounds is crucial for applications in chemistry, pharmaceuticals, and materials science.
Key Differences Between Saturated and Anhydrous States
Saturated compounds contain the maximum number of hydrogen atoms bonded to carbon atoms with no double or triple bonds, whereas anhydrous substances are free from water molecules in their crystalline or solid state. The key differences between saturated and anhydrous states lie in saturation referring to chemical bonding saturation in molecules, while anhydrous pertains to the absence of water content in a material or compound. Saturation affects molecular structure and reactivity, whereas anhydrous status influences physical properties like hygroscopicity and stability during storage.
Chemical Definitions: Saturated Explained
Saturated compounds contain the maximum number of hydrogen atoms bonded to carbon atoms, resulting in only single bonds within their molecular structure. This chemical definition highlights that saturated molecules, such as saturated hydrocarbons, lack double or triple carbon-carbon bonds, making them chemically stable and less reactive. Anhydrous compounds, by contrast, are substances that contain no water molecules, emphasizing the absence of water rather than saturation in chemical bonding.
What Does Anhydrous Mean in Chemistry?
Anhydrous in chemistry refers to a substance that contains no water molecules, either free or chemically bound. This term is crucial for chemicals like salts or solvents, where the presence of water can alter reactivity, stability, and purity. Understanding the difference between anhydrous and hydrated forms enables precise control in chemical reactions and formulations.
Molecular Structure: Saturated vs Anhydrous
Saturated compounds contain molecules in which all carbon atoms are bonded by single covalent bonds, resulting in a stable, fully "saturated" structure with hydrogen atoms filling all available bonding sites. Anhydrous substances refer to compounds that lack water molecules in their molecular structure, often resulting in more concentrated forms without hydration shells. The molecular distinction lies in saturated molecules' complete hydrogen bonding within the carbon chain, while anhydrous forms emphasize absence of water rather than bond saturation.
Physical Properties Comparison
Saturated compounds typically exhibit higher melting and boiling points due to the absence of double or triple bonds, resulting in stronger intermolecular forces. Anhydrous substances lack water molecules, which often leads to increased density and a more crystalline structure compared to their hydrated counterparts. The physical state and solubility of saturated versus anhydrous materials differ significantly, impacting their stability and behavior under varying temperature and humidity conditions.
Common Examples of Saturated and Anhydrous Substances
Common examples of saturated substances include saturated fats like stearic acid and palmitic acid, which contain no double bonds in their hydrocarbon chains. Anhydrous substances often refer to compounds like anhydrous calcium sulfate (plaster of Paris) and anhydrous sodium carbonate, both of which lack water molecules that are typically present in their hydrated forms. The distinction between saturated and anhydrous substances is critical in chemistry and industrial applications due to differences in molecular structure and physical properties.
Industrial Applications: Choosing the Right Form
Saturated and anhydrous forms are critical in industrial applications, where the choice impacts product stability and process efficiency. Saturated solutions, containing the maximum dissolved solute at a given temperature, are ideal for crystallization and extraction processes, ensuring consistent concentration and reaction rates. Anhydrous compounds, devoid of water, are preferred in pharmaceutical manufacturing and chemical synthesis to prevent hydrolysis and maintain reagent purity.
Impacts on Chemical Reactions and Solubility
Saturated compounds contain the maximum number of hydrogen atoms, which limits their reactivity due to the absence of double or triple bonds, while anhydrous substances lack water molecules, often enhancing their purity and reactivity in chemical reactions. The presence of water in hydrated compounds can significantly affect solubility and reaction rates, as anhydrous forms typically dissolve differently and may participate in faster or more selective reactions. Understanding the saturated versus anhydrous state is crucial in predicting solubility behavior and reaction mechanisms in organic and inorganic chemistry.
Safety and Storage Considerations
Saturated solutions contain dissolved solutes up to their maximum capacity, requiring careful storage to prevent precipitation and maintain stability, while anhydrous substances lack water, reducing risks of hydrolysis but necessitating moisture-proof containers to avoid clumping or degradation. Proper labeling and storage in airtight, dry conditions are critical for anhydrous materials to ensure safety and product integrity. Safety measures must account for the potential hazards associated with both forms, including chemical reactivity and environmental sensitivity.
Saturated Infographic
 libterm.com
libterm.com