Ketose sugars, characterized by a ketone group within their structure, play a vital role in various biochemical processes and metabolism. Unlike aldoses, ketoses such as fructose serve as important energy sources and intermediates in carbohydrate pathways. Explore the article to understand the significance of ketoses in your diet and metabolism.
Table of Comparison
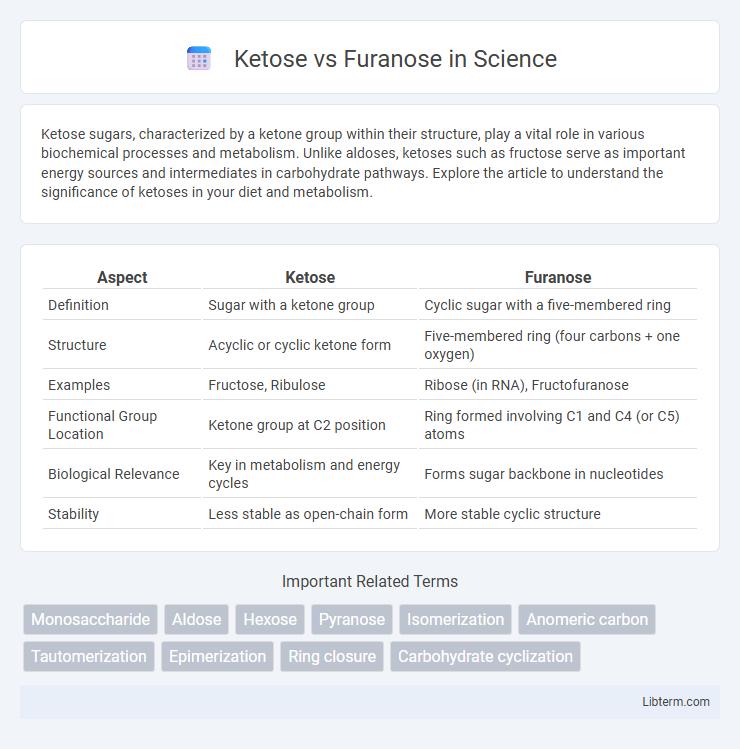
| Aspect | Ketose | Furanose |
|---|---|---|
| Definition | Sugar with a ketone group | Cyclic sugar with a five-membered ring |
| Structure | Acyclic or cyclic ketone form | Five-membered ring (four carbons + one oxygen) |
| Examples | Fructose, Ribulose | Ribose (in RNA), Fructofuranose |
| Functional Group Location | Ketone group at C2 position | Ring formed involving C1 and C4 (or C5) atoms |
| Biological Relevance | Key in metabolism and energy cycles | Forms sugar backbone in nucleotides |
| Stability | Less stable as open-chain form | More stable cyclic structure |
Introduction to Ketose and Furanose
Ketose refers to a monosaccharide containing a ketone functional group, typically found in the second carbon atom, playing a crucial role in carbohydrate metabolism. Furanose denotes a five-membered ring structure formed when a sugar's carbonyl group reacts with a hydroxyl group within the same molecule, common in both ketoses and aldoses. The distinction between ketose and furanose lies in ketose's linear ketone form and furanose's cyclic ring conformation, essential for understanding sugar chemistry and biochemical pathways.
Structural Differences: Ketose vs Furanose
Ketose sugars contain a ketone functional group typically at the second carbon atom, distinguishing them structurally from furanose forms, which are five-membered ring structures consisting of four carbons and one oxygen atom. The ketose form is an open-chain or linear structure before cyclization, while the furanose form results from the intramolecular reaction between a hydroxyl group and the carbonyl carbon, creating a cyclic hemiacetal or hemiketal. This ring formation significantly alters the sugar's chemical reactivity and physical properties compared to the ketose's linear structure.
Chemical Properties Comparison
Ketose and furanose differ distinctly in their chemical structures and reactivity; ketoses contain a keto group typically at the second carbon atom, resulting in a more reactive carbonyl center compared to aldoses, while furanoses are cyclic forms of sugars featuring a five-membered ring composed of four carbons and one oxygen atom. Ketose sugars readily undergo enolization due to the presence of an a-hydrogen adjacent to the keto group, influencing their behavior in biochemical pathways and reactions such as the Maillard reaction. Furanose forms exhibit unique ring strain and conformational flexibility, affecting their stability, reactivity in glycosidic bond formation, and susceptibility to enzymatic hydrolysis compared to their open-chain ketose counterparts.
Occurrence in Nature
Ketose sugars commonly occur in nature as furanose forms, especially in five-carbon monosaccharides like fructose, which frequently adopts a furanose ring structure. Furanose forms are prevalent in ketose sugars due to the intramolecular reaction between the ketone group and the hydroxyl group on the fourth carbon, forming a stable five-membered ring. In contrast, aldose sugars typically favor pyranose ring forms, emphasizing the distinctive occurrence patterns of ketose in natural carbohydrate structures.
Roles in Carbohydrate Chemistry
Ketose and furanose structures play crucial roles in carbohydrate chemistry by influencing molecular reactivity and biological function. Ketoses contain a ketone group that enables isomerization and participation in metabolic pathways such as glycolysis, while furanoses, five-membered ring forms of sugars, impact carbohydrate stability and recognition by enzymes. Understanding these forms aids in deciphering carbohydrate behavior in energy storage, signaling, and structural roles.
Metabolic Pathways Involving Ketoses and Furanoses
Ketoses and furanoses play distinct roles in metabolic pathways, with ketoses primarily involved in glycolysis and fructose metabolism as key intermediates like fructose-6-phosphate and dihydroxyacetone phosphate. Furanoses, five-membered ring sugar forms, are crucial in nucleotide biosynthesis and RNA structure, prominently seen in ribose-5-phosphate within the pentose phosphate pathway. Metabolic enzymes such as isomerases and kinases facilitate the interconversion between ketose and furanose forms, optimizing cellular energy production and biosynthetic precursor availability.
Biological Significance and Functions
Ketose sugars, characterized by a ketone group typically at the second carbon, play crucial roles in metabolic pathways such as glycolysis and the pentose phosphate pathway, providing essential intermediates like dihydroxyacetone phosphate. Furanose forms, five-membered ring structures found in many ribose sugars, are vital components of nucleotides, forming the sugar backbone of RNA, which is essential for genetic information transmission and cellular function. The structural differences between ketose and furanose forms influence their reactivity and interaction with enzymes, impacting energy production, nucleic acid stability, and overall cellular metabolism.
Analytical Methods for Differentiation
Ketose and furanose forms can be effectively differentiated using Nuclear Magnetic Resonance (NMR) spectroscopy, which provides distinct chemical shift patterns for the anomeric protons and carbon atoms. Mass Spectrometry (MS) coupled with chromatographic techniques, such as Liquid Chromatography-Mass Spectrometry (LC-MS), enables separation and identification based on molecular masses and fragmentation patterns unique to ketose and furanose structures. Infrared (IR) spectroscopy, particularly Fourier Transform Infrared (FTIR), assists in detecting characteristic functional group vibrations, aiding in distinguishing the ketone group of ketoses from the ether ring of furanoses.
Industrial and Biotechnological Applications
Ketose and furanose forms differ significantly in their industrial and biotechnological applications due to their structural properties influencing reactivity and stability. Ketoses, commonly found in their linear form or as furanoses, are essential in fermentation processes and as precursors for biofuels and value-added chemicals like hydroxymethylfurfural (HMF). Furanoses, with their five-membered ring structure, are crucial in pharmaceutical drug design and enzymatic synthesis, offering enhanced specificity and functionality in biomolecular engineering.
Summary: Key Differences and Similarities
Ketose and furanose are carbohydrate structures distinguished by their functional groups and ring formation; ketose contains a ketone group while furanose refers to a five-membered ring structure commonly formed by sugars like fructose. Both ketose and furanose play crucial roles in carbohydrate chemistry and metabolism, with ketoses often cyclizing into furanose rings or pyranose rings depending on the sugar and conditions. Key similarities include their involvement in sugar isomerism and energy metabolism, while their key difference lies in ketose's functional group as a carbonyl compound and furanose's specific cyclic ring conformation.
Ketose Infographic
 libterm.com
libterm.com