Electrolytes are substances that dissociate into ions when dissolved in water, allowing the solution to conduct electricity. Conductors, on the other hand, are materials like metals that permit electric current to flow freely through their structure without ionization. Discover how understanding the differences between electrolytes and conductors can enhance Your knowledge in electronics by reading the rest of the article.
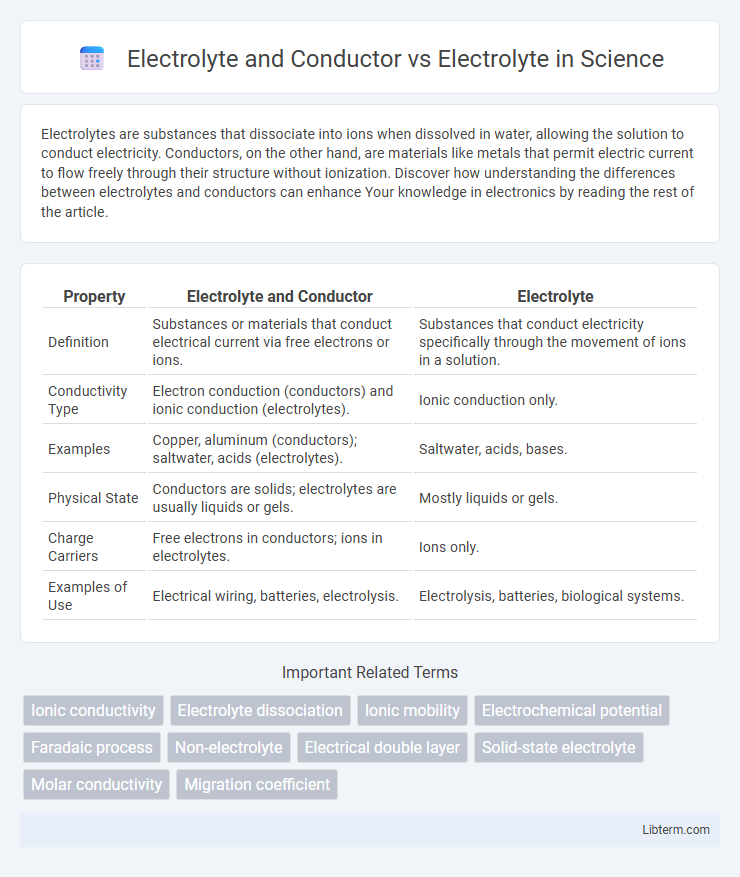
Table of Comparison
| Property | Electrolyte and Conductor | Electrolyte |
|---|---|---|
| Definition | Substances or materials that conduct electrical current via free electrons or ions. | Substances that conduct electricity specifically through the movement of ions in a solution. |
| Conductivity Type | Electron conduction (conductors) and ionic conduction (electrolytes). | Ionic conduction only. |
| Examples | Copper, aluminum (conductors); saltwater, acids (electrolytes). | Saltwater, acids, bases. |
| Physical State | Conductors are solids; electrolytes are usually liquids or gels. | Mostly liquids or gels. |
| Charge Carriers | Free electrons in conductors; ions in electrolytes. | Ions only. |
| Examples of Use | Electrical wiring, batteries, electrolysis. | Electrolysis, batteries, biological systems. |
Introduction to Electrolytes and Conductors
Electrolytes are substances that dissociate into ions when dissolved in a solvent, enabling the conduction of electric current through ionic movement. Conductors, in contrast, rely on the flow of free electrons within a solid or liquid medium to transmit electricity. Understanding the distinction between ionic conduction in electrolytes and electronic conduction in conductors is fundamental to applications in batteries, fuel cells, and electrochemical sensors.
What Is an Electrolyte?
An electrolyte is a substance that dissociates into ions when dissolved in a solvent, enabling the solution to conduct electricity by allowing ion movement. Unlike conductors, which conduct electricity through the flow of electrons, electrolytes facilitate electrical conduction through charged ions in liquids or gels. Common electrolytes include sodium chloride, potassium ions, and calcium ions, essential for processes such as nerve impulse transmission and muscle contraction.
Characteristics of Conductors
Conductors exhibit high electrical conductivity due to the presence of free-moving electrons within their atomic structure, enabling efficient current flow with minimal resistance. Unlike electrolytes, which rely on ion mobility in solution or molten state, conductors maintain electron mobility in solid form, resulting in superior electrical performance. Key characteristics include low resistivity, rapid response to electric fields, and stability under varying temperatures.
Types of Electrolytes
Electrolytes are substances that dissociate into ions when dissolved in a solvent, enabling the conduction of electric current, whereas electrolytes combined with conductors facilitate improved electrical pathways in devices like batteries and fuel cells. Types of electrolytes include strong electrolytes, such as sodium chloride and potassium hydroxide, which fully dissociate in solution, and weak electrolytes, like acetic acid, which partially dissociate, affecting the solution's conductivity. Solid, liquid, and gel electrolytes represent different physical states used depending on the application, with solid polymer electrolytes gaining prominence in advanced lithium-ion batteries for enhanced safety and performance.
Differences Between Electrolytes and Conductors
Electrolytes are substances that produce ions in solution, enabling electrical conductivity through ion migration, whereas conductors allow electron flow without ion movement. Electrolyte conductivity depends on ion concentration and mobility, while conductors rely on free electrons in metallic or conductive materials. Unlike solid conductors, electrolytes typically exist as liquids or gels facilitating ionic conduction in applications like batteries and fuel cells.
How Electrolytes Conduct Electricity
Electrolytes conduct electricity by dissociating into ions when dissolved in a solvent, allowing the free movement of charged particles that carry electrical current through the solution. Unlike conductors such as metals, which rely on free electrons for conductivity, electrolytes depend on ion mobility within the liquid or gel medium to facilitate electrical flow. The efficiency of an electrolyte in conducting electricity is influenced by ion concentration, temperature, and the type of solvent used.
Factors Affecting Electrolyte Conductivity
Electrolyte and conductor materials exhibit distinct conductivity influenced primarily by ion concentration, temperature, and the mobility of charge carriers within the electrolyte solution. High ion concentration increases electrolyte conductivity by providing more charge carriers, while temperature elevation enhances ion mobility, thus improving conduction efficiency. The presence of impurities or solvent viscosity can significantly reduce electrolyte conductivity by hindering ion flow in both electrolyte and conductor systems.
Common Examples of Electrolytes and Conductors
Common examples of electrolytes include sodium chloride (NaCl) dissolved in water, potassium hydroxide (KOH), and hydrochloric acid (HCl), which dissociate into ions facilitating electrical conductivity. Conductors primarily consist of metals such as copper, silver, and aluminum, where free electrons enable efficient electric current flow without the requirement of ionic dissociation. Electrolytes conduct electricity through ion movement in solution, whereas conductors rely on free electron mobility within solid materials.
Electrolyte vs. Non-Electrolyte: Key Contrasts
Electrolytes are substances that dissociate into ions when dissolved in water, enabling the solution to conduct electricity, whereas non-electrolytes do not dissociate and therefore do not conduct electricity. The primary distinction lies in the presence of free ions in electrolytes, which act as charge carriers, compared to non-electrolytes that remain as intact molecules. This fundamental difference impacts applications in batteries, biological systems, and industrial processes where ion conduction is critical.
Applications and Importance in Everyday Life
Electrolytes enable ionic conduction in solutions, essential for biological functions like nerve signaling and hydration, while conductors transmit electrical current via free electrons, crucial for powering electronic devices and wiring systems. Electrolyte solutions are vital in batteries, supporting energy storage and release during charging and discharging cycles, whereas solid conductors form the backbone of electrical circuits and power grids. Their complementary roles in energy transfer and electrical conduction underpin technologies ranging from medical diagnostics to renewable energy systems, emphasizing their importance in everyday life.
Electrolyte and Conductor Infographic
 libterm.com
libterm.com