Atomic energy harnesses the power of atomic nuclei through processes like fission and fusion to generate vast amounts of electricity with minimal carbon emissions. This technology plays a crucial role in providing reliable and sustainable energy solutions worldwide, balancing environmental concerns with growing power demands. Explore the rest of the article to understand how atomic energy impacts your future and the global energy landscape.
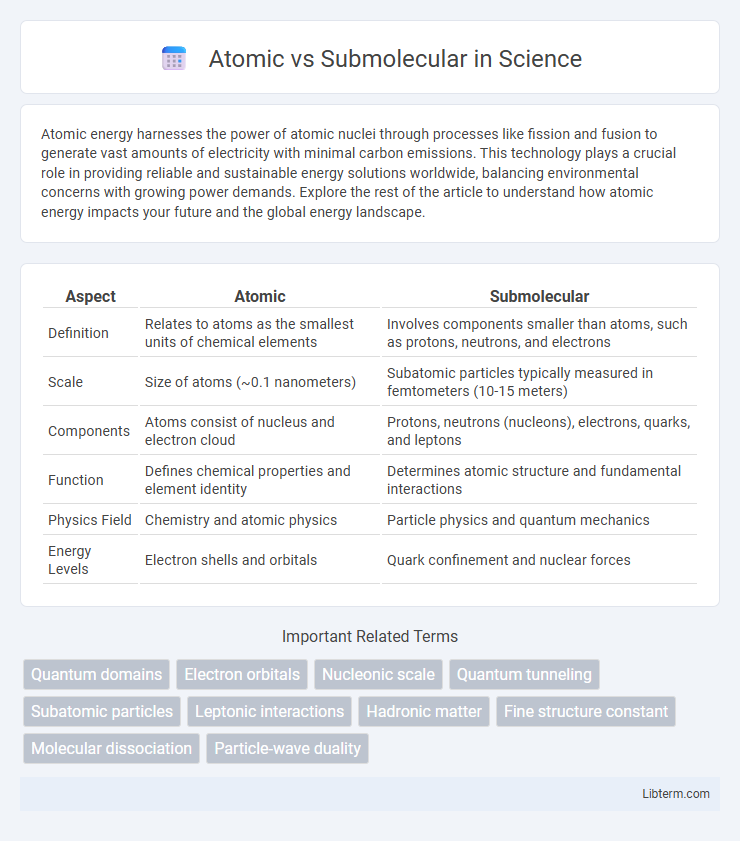
Table of Comparison
| Aspect | Atomic | Submolecular |
|---|---|---|
| Definition | Relates to atoms as the smallest units of chemical elements | Involves components smaller than atoms, such as protons, neutrons, and electrons |
| Scale | Size of atoms (~0.1 nanometers) | Subatomic particles typically measured in femtometers (10-15 meters) |
| Components | Atoms consist of nucleus and electron cloud | Protons, neutrons (nucleons), electrons, quarks, and leptons |
| Function | Defines chemical properties and element identity | Determines atomic structure and fundamental interactions |
| Physics Field | Chemistry and atomic physics | Particle physics and quantum mechanics |
| Energy Levels | Electron shells and orbitals | Quark confinement and nuclear forces |
Introduction to Atomic and Submolecular Scales
Atomic scales measure the size and behavior of atoms, typically ranging from 0.1 to 0.5 nanometers, encompassing protons, neutrons, and electrons. Submolecular scales delve deeper into the components within atoms, including quarks and gluons that form protons and neutrons, existing at femtometer dimensions (10^-15 meters). Understanding the distinction between atomic and submolecular scales is essential for exploring fundamental particle physics and quantum mechanics.
Defining Atomic Structure
Atomic structure defines the arrangement of protons, neutrons, and electrons within an atom, determining its chemical properties and identity. Submolecular particles, such as quarks and gluons, compose protons and neutrons, representing the fundamental constituents beyond the atomic scale. Understanding atomic structure bridges the gap between observable chemical behavior and the submolecular interactions governing particle physics.
Understanding Submolecular Entities
Submolecular entities represent structures smaller than atoms, encompassing particles like electrons, protons, neutrons, and quarks that define atomic composition and properties. Understanding submolecular entities involves studying their behavior, interactions, and roles within atomic nuclei and electron clouds, which directly influence chemical bonding and physical characteristics. Advances in quantum mechanics and particle physics provide detailed insights into these fundamental components, enabling precise modeling of atomic-scale phenomena and material properties.
Key Differences Between Atomic and Submolecular Levels
Atomic level refers to the scale of individual atoms, which are the fundamental units of chemical elements consisting of protons, neutrons, and electrons. Submolecular level involves structures smaller than an atom, such as quarks and gluons, which form protons and neutrons within the atomic nucleus. Key differences include the atomic level's focus on element identity and chemical properties, while the submolecular level addresses the fundamental particles and forces that constitute atomic components.
Chemical Bonding: Atomic vs Submolecular Interactions
Chemical bonding involves atomic interactions where atoms share or transfer electrons to form molecules, exhibiting ionic, covalent, or metallic bonds. Submolecular interactions, including van der Waals forces, hydrogen bonds, and dipole-dipole interactions, occur between parts of molecules or subunits, influencing molecular stability and properties without forming new bonds. Understanding the distinction between atomic and submolecular bonding mechanisms is crucial for manipulating material properties and biochemical processes.
Role in Material Properties
Atomic structure defines the fundamental arrangement of protons, neutrons, and electrons, directly influencing a material's macroscopic properties such as conductivity, magnetism, and mechanical strength. Submolecular elements, including molecular orbitals and electron clouds, govern finer interactions like chemical bonding, electron delocalization, and intermolecular forces, which critically determine a material's reactivity, flexibility, and optical characteristics. Understanding both atomic and submolecular roles enables precise manipulation of material properties for advanced applications in nanotechnology and material science.
Analytical Techniques for Atomic and Submolecular Observation
Atomic observation techniques primarily rely on spectroscopy methods such as X-ray fluorescence (XRF) and atomic absorption spectroscopy (AAS) that analyze atomic energy levels and elemental composition. Submolecular observation demands advanced methods like scanning tunneling microscopy (STM) and atomic force microscopy (AFM), which provide spatial resolution at the scale of individual molecular bonds and electron distributions. These analytical techniques enable detailed characterization from atomic elements to the intricate features within molecules, enhancing understanding in materials science and nanotechnology.
Applications in Nanotechnology and Molecular Engineering
Atomic-level manipulation enables precise control over matter, crucial for fabricating nanoscale devices with desired electronic, optical, and mechanical properties in nanotechnology. Submolecular engineering delves deeper into tuning chemical bonds and electron distributions within molecules, enhancing molecular machines and targeted drug delivery systems. These approaches collectively advance molecular engineering by allowing tailored design and assembly of functional nanomaterials with unprecedented accuracy.
Implications for Quantum Mechanics
Atomic structures serve as the foundational basis for quantum mechanics, where electron orbitals and energy levels define the probabilistic nature of particles. Submolecular components, such as quarks and gluons within protons and neutrons, extend quantum theory into quantum chromodynamics, revealing deeper layers of particle interactions and confinement. Understanding these distinctions enhances models of particle behavior, refining predictions of quantum states and fundamental forces.
Future Perspectives in Atomic and Submolecular Research
Future perspectives in atomic and submolecular research emphasize advancements in atomic-scale manipulation and quantum control techniques, unlocking unprecedented precision in materials science and nanotechnology. Emerging technologies in submolecular spectroscopy and electron microscopy are enabling insights into molecular interactions at the electron orbital level, paving the way for innovative drug design and energy storage solutions. Integration of artificial intelligence with atomic and submolecular data accelerates discovery, fostering breakthroughs in quantum computing and catalysis.
Atomic Infographic
 libterm.com
libterm.com