Differentiation is a fundamental concept in calculus that measures the rate at which a function changes with respect to its variable, providing critical insights into motion, growth, and optimization problems. The derivative, representing this rate of change, is essential for understanding slopes of curves and solving real-world problems in physics, engineering, and economics. Explore the rest of this article to deepen your understanding of differentiation and its practical applications.
Table of Comparison
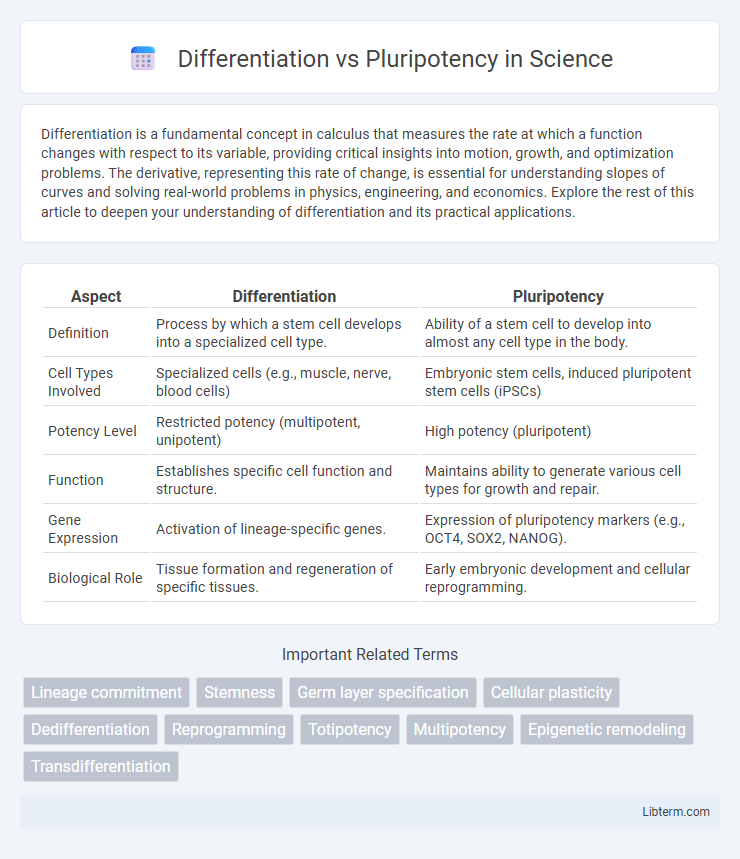
| Aspect | Differentiation | Pluripotency |
|---|---|---|
| Definition | Process by which a stem cell develops into a specialized cell type. | Ability of a stem cell to develop into almost any cell type in the body. |
| Cell Types Involved | Specialized cells (e.g., muscle, nerve, blood cells) | Embryonic stem cells, induced pluripotent stem cells (iPSCs) |
| Potency Level | Restricted potency (multipotent, unipotent) | High potency (pluripotent) |
| Function | Establishes specific cell function and structure. | Maintains ability to generate various cell types for growth and repair. |
| Gene Expression | Activation of lineage-specific genes. | Expression of pluripotency markers (e.g., OCT4, SOX2, NANOG). |
| Biological Role | Tissue formation and regeneration of specific tissues. | Early embryonic development and cellular reprogramming. |
Introduction to Differentiation and Pluripotency
Differentiation is the biological process where unspecialized cells develop into distinct cell types with specific functions, guided by gene expression and environmental cues. Pluripotency refers to the capacity of stem cells to differentiate into nearly all cell types of the body, maintaining high developmental potential. Understanding the molecular mechanisms regulating pluripotency and differentiation is crucial for advancements in regenerative medicine and developmental biology.
Defining Cellular Differentiation
Cellular differentiation is the biological process where a less specialized cell develops into a more specialized cell type with distinct functions, enabling the formation of various tissues and organs. This process involves changes in gene expression patterns and epigenetic modifications that restrict the cell's developmental potential compared to pluripotency. Unlike pluripotent stem cells, which can give rise to all cell types in the body, differentiated cells exhibit a specific phenotype essential for maintaining tissue-specific functions.
Understanding Pluripotency in Stem Cells
Pluripotency in stem cells refers to the ability to differentiate into any cell type of the three germ layers: ectoderm, mesoderm, and endoderm, enabling extensive cellular diversity. This characteristic is regulated by core transcription factors such as OCT4, SOX2, and NANOG, which maintain the undifferentiated state and self-renewal capacity. Understanding pluripotency is crucial for advancing regenerative medicine, as it provides the foundation for developing therapies based on controlled differentiation into specific cell lineages.
Key Molecular Markers of Differentiation and Pluripotency
Key molecular markers of pluripotency include OCT4, SOX2, and NANOG, which maintain the self-renewal and undifferentiated state of stem cells. Differentiation is characterized by the downregulation of pluripotency markers and the expression of lineage-specific markers such as GATA4 for endoderm, Brachyury (T) for mesoderm, and PAX6 for ectoderm. The dynamic regulation of these markers directs stem cells toward specific developmental fates and functional cell types.
Mechanisms Regulating Differentiation
Mechanisms regulating differentiation involve intricate signaling pathways such as Notch, Wnt, and BMP that guide stem cells toward specialized cell fates by modulating gene expression and epigenetic landscapes. Transcription factors like Oct4, Sox2, and Nanog maintain pluripotency by activating pluripotency-associated genes and repressing differentiation-specific genes, ensuring self-renewal capability. Epigenetic modifications, including DNA methylation and histone acetylation, play a critical role in stabilizing differentiated states by altering chromatin accessibility and silencing pluripotency genes.
Pathways Sustaining Pluripotency
Pathways sustaining pluripotency primarily involve the activation of core transcription factors such as Oct4, Sox2, and Nanog, which maintain the self-renewal capacity of embryonic stem cells. Key signaling pathways including the LIF/STAT3, Wnt/b-catenin, and PI3K/Akt cascades contribute to the stabilization of this pluripotent state by inhibiting differentiation signals. Epigenetic regulators such as histone modifications and DNA methylation patterns further preserve the pluripotent chromatin landscape, preventing lineage-specific gene expression and promoting cellular plasticity.
Differentiation vs Pluripotency: Biological Significance
Differentiation is the process by which pluripotent stem cells develop into specialized cell types, enabling the formation of diverse tissues and organs essential for organismal function. Pluripotency confers the ability of stem cells to self-renew and give rise to nearly all cell lineages, maintaining developmental potential during early embryogenesis. The balance between differentiation and pluripotency is critical for proper tissue development, regeneration, and homeostasis, with disruptions implicated in developmental disorders and cancer.
Experimental Techniques for Assessing Cell States
Experimental techniques for assessing differentiation versus pluripotency include flow cytometry, which quantifies cell surface markers like TRA-1-60 and SSEA-4 specific to pluripotent stem cells. Immunocytochemistry enables visualization of lineage-specific proteins, such as SOX2 for pluripotency or TUJ1 for neural differentiation, providing spatial context within cells. Quantitative PCR and RNA sequencing offer high-resolution data on gene expression profiles, distinguishing pluripotent cells from differentiated progeny through transcriptional signatures.
Clinical Implications and Therapeutic Applications
Differentiation guides stem cells to develop into specialized cell types, essential for targeted cell replacement therapies in conditions like Parkinson's disease and myocardial infarction. Pluripotency allows stem cells to generate any cell type, offering vast potential for regenerative medicine but posing risks such as teratoma formation in clinical applications. Understanding the balance between maintaining pluripotency and promoting controlled differentiation is critical for optimizing safe and effective stem cell therapies.
Future Directions in Stem Cell Research
Future directions in stem cell research prioritize harnessing the balance between differentiation and pluripotency to optimize regenerative medicine outcomes. Advances in single-cell RNA sequencing and CRISPR-based gene editing are enabling precise manipulation of pluripotent stem cells to direct their differentiation pathways for tissue-specific repair. Emerging technologies such as organoid development and synthetic niches aim to mimic in vivo environments, enhancing pluripotent stem cell maintenance and controlled differentiation for therapeutic applications.
Differentiation Infographic
 libterm.com
libterm.com