A weak electrolyte partially ionizes in solution, resulting in a lower concentration of ions compared to strong electrolytes. This limited ionization affects the solution's electrical conductivity, making it less conductive. Explore the rest of the article to understand how weak electrolytes impact chemical reactions and real-world applications.
Table of Comparison
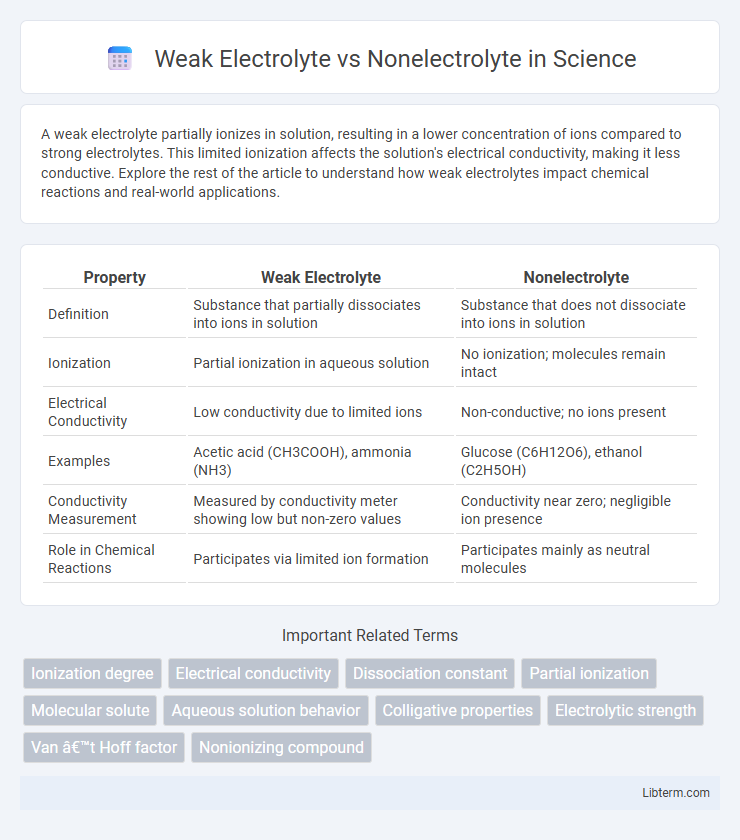
| Property | Weak Electrolyte | Nonelectrolyte |
|---|---|---|
| Definition | Substance that partially dissociates into ions in solution | Substance that does not dissociate into ions in solution |
| Ionization | Partial ionization in aqueous solution | No ionization; molecules remain intact |
| Electrical Conductivity | Low conductivity due to limited ions | Non-conductive; no ions present |
| Examples | Acetic acid (CH3COOH), ammonia (NH3) | Glucose (C6H12O6), ethanol (C2H5OH) |
| Conductivity Measurement | Measured by conductivity meter showing low but non-zero values | Conductivity near zero; negligible ion presence |
| Role in Chemical Reactions | Participates via limited ion formation | Participates mainly as neutral molecules |
Introduction to Electrolytes
Weak electrolytes partially ionize in water, producing a limited number of ions that conduct electricity moderately. Nonelectrolytes do not ionize in solution and therefore do not conduct electricity. The degree of ionization distinguishes weak electrolytes from nonelectrolytes, affecting their electrical conductivity and chemical behavior in aqueous solutions.
Defining Weak Electrolytes
Weak electrolytes are substances that partially dissociate into ions in aqueous solution, resulting in a limited electrical conductivity compared to strong electrolytes. Common examples of weak electrolytes include acetic acid (CH3COOH) and ammonia (NH3), which only ionize to a small extent, producing a dynamic equilibrium between ions and undissociated molecules. This partial ionization differentiates weak electrolytes from nonelectrolytes, which do not ionize at all in water and therefore do not conduct electricity.
What Are Nonelectrolytes?
Nonelectrolytes are substances that do not dissociate into ions when dissolved in water, resulting in no electrical conductivity in the solution. Common examples include sugar and ethanol, which remain as intact molecules in solution. Unlike weak electrolytes, nonelectrolytes do not partially ionize and therefore cannot conduct electricity.
Key Differences: Weak Electrolyte vs Nonelectrolyte
Weak electrolytes partially dissociate into ions in solution, resulting in limited electrical conductivity, while nonelectrolytes do not dissociate into ions and therefore do not conduct electricity. Examples of weak electrolytes include acetic acid and ammonia, which produce fewer ions compared to strong electrolytes. Nonelectrolytes like sugar and ethanol dissolve without ionization, maintaining molecular integrity and exhibiting no conductivity in aqueous solutions.
Common Examples of Weak Electrolytes
Weak electrolytes partially dissociate into ions in aqueous solutions, resulting in limited electrical conductivity, with common examples including acetic acid (CH3COOH), ammonia (NH3), and hydrogen fluoride (HF). These substances unlike strong electrolytes do not fully ionize, maintaining a dynamic equilibrium between the molecular and ionic forms. Nonelectrolytes such as glucose and urea remain largely in molecular form without producing ions, thus exhibiting negligible conductivity.
Common Nonelectrolyte Substances
Common nonelectrolyte substances include sugar, ethanol, and urea, which do not dissociate into ions when dissolved in water, resulting in no electrical conductivity. Weak electrolytes, such as acetic acid and ammonia, partially ionize in solution, producing a limited amount of ions and moderate conductivity. Unlike weak electrolytes, nonelectrolytes remain intact as molecules, making them important in applications where electrical neutrality and non-conductivity are required.
Ionization in Solutions: Weak Electrolytes vs Nonelectrolytes
Weak electrolytes partially ionize in solution, producing a limited number of ions that conduct electricity weakly, whereas nonelectrolytes do not ionize and remain as intact molecules, resulting in no electrical conductivity. The degree of ionization of weak electrolytes depends on factors such as concentration and temperature, often represented by an equilibrium expression for their dissociation. In contrast, nonelectrolytes exhibit no measurable ionization constant, reflecting their complete lack of ion formation in solution.
Conductivity Comparison: Weak Electrolytes and Nonelectrolytes
Weak electrolytes partially ionize in solution, resulting in limited electrical conductivity, whereas nonelectrolytes do not ionize and thus exhibit negligible conductivity. The degree of ionization in weak electrolytes directly influences the concentration of ions available to conduct electricity, making their conductivity higher than nonelectrolytes but significantly lower than strong electrolytes. Common examples include acetic acid as a weak electrolyte and glucose as a nonelectrolyte, highlighting their distinct behaviors in aqueous solutions.
Real-World Applications and Importance
Weak electrolytes partially dissociate into ions in solution, making them essential in medical treatments like intravenous fluids where controlled ion release maintains electrolyte balance. Nonelectrolytes, which do not ionize, are vital in industries such as pharmaceuticals and food processing where non-conductive solvents ensure stability and purity. Understanding the distinction influences the formulation of batteries, drug delivery systems, and environmental monitoring technologies.
Summary: Choosing Between Weak Electrolytes and Nonelectrolytes
Weak electrolytes partially dissociate into ions in solution, allowing limited electrical conductivity, while nonelectrolytes do not dissociate and thus do not conduct electricity. Selecting between weak electrolytes and nonelectrolytes depends on the desired conductivity and reactivity in chemical processes, such as in buffer solutions or non-conductive solvents. Understanding their ionization properties aids in optimizing applications in electrochemistry and solution chemistry.
Weak Electrolyte Infographic
 libterm.com
libterm.com