Constitutional isomers are compounds with the same molecular formula but different connectivity of their atoms, resulting in distinct structures and properties. Understanding these differences is essential for grasping chemical behavior and reactivity. Explore the rest of the article to dive deeper into the fascinating world of constitutional isomers and their significance.
Table of Comparison
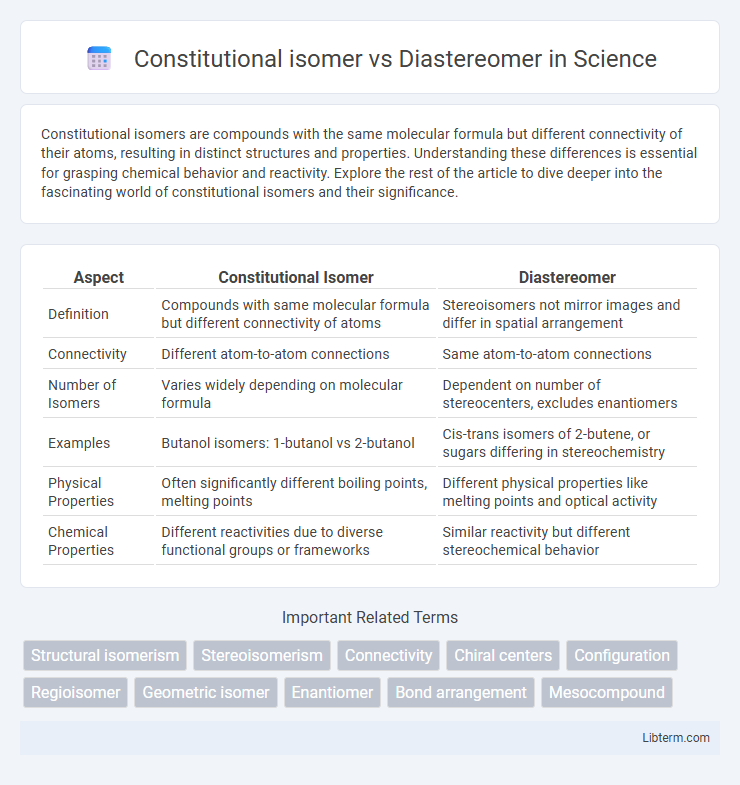
| Aspect | Constitutional Isomer | Diastereomer |
|---|---|---|
| Definition | Compounds with same molecular formula but different connectivity of atoms | Stereoisomers not mirror images and differ in spatial arrangement |
| Connectivity | Different atom-to-atom connections | Same atom-to-atom connections |
| Number of Isomers | Varies widely depending on molecular formula | Dependent on number of stereocenters, excludes enantiomers |
| Examples | Butanol isomers: 1-butanol vs 2-butanol | Cis-trans isomers of 2-butene, or sugars differing in stereochemistry |
| Physical Properties | Often significantly different boiling points, melting points | Different physical properties like melting points and optical activity |
| Chemical Properties | Different reactivities due to diverse functional groups or frameworks | Similar reactivity but different stereochemical behavior |
Introduction to Constitutional Isomers and Diastereomers
Constitutional isomers are compounds with the same molecular formula but different connectivity of atoms, resulting in distinct structural frameworks. Diastereomers are stereoisomers that are not mirror images and differ in the spatial arrangement of atoms at one or more stereocenters, excluding enantiomeric relationships. Understanding these differences is essential in organic chemistry to predict chemical properties and reactivity.
Foundational Concepts in Structural Isomerism
Constitutional isomers differ in the connectivity of atoms within their molecular structures, resulting in variations in the sequence of bonded atoms and overall molecular framework. Diastereomers are stereoisomers that share the same connectivity but differ in the spatial arrangement of atoms at one or more stereocenters, leading to non-mirror-image configurations. Understanding these foundational concepts in structural isomerism is crucial for distinguishing between differences in molecular connectivity and stereochemical orientation.
Constitutional Isomers: Definition and Characteristics
Constitutional isomers, also known as structural isomers, are compounds with the same molecular formula but different connectivity of atoms, resulting in varied physical and chemical properties. These isomers differ in the order in which atoms are bonded, leading to distinct functional groups or carbon skeletons within molecules. Unlike diastereomers, constitutional isomers do not involve stereochemical differences but are defined purely by their unique atomic arrangements.
Diastereomers: Definition and Characteristics
Diastereomers are stereoisomers that are not mirror images of each other and differ in the spatial arrangement of atoms at one or more stereocenters. They exhibit different physical and chemical properties, such as melting points, boiling points, and reactivity, making them distinguishable in laboratory conditions. Unlike constitutional isomers, which differ in connectivity of atoms, diastereomers share the same molecular formula and connectivity but vary in their stereochemistry.
Key Differences Between Constitutional Isomers and Diastereomers
Constitutional isomers differ in the connectivity of their atoms, resulting in distinct molecular structures and different physical and chemical properties, despite having the same molecular formula. Diastereomers, on the other hand, share the same atomic connectivity but differ in the spatial arrangement of atoms at one or more stereocenters, leading to differences in stereochemistry without changes in molecular formula. Key distinctions include that constitutional isomers vary in bonding patterns while diastereomers vary in stereochemistry, impacting their reactivity and interaction with other molecules.
Structural Representation and Identification Techniques
Constitutional isomers differ in the connectivity of atoms, leading to distinct structural formulas that can be identified using techniques like Nuclear Magnetic Resonance (NMR) spectroscopy and mass spectrometry, which reveal differences in bonding and molecular fragments. Diastereomers share the same connectivity but vary in spatial arrangement at one or more stereocenters, requiring chiral resolution techniques or advanced NMR methods such as NOESY to differentiate them. X-ray crystallography provides precise three-dimensional structural representation, crucial for distinguishing both constitutional isomers and diastereomers at the atomic level.
Physical and Chemical Properties Comparison
Constitutional isomers differ in connectivity, leading to distinct physical properties such as boiling points, melting points, and densities due to variations in molecular structure and intermolecular forces. Diastereomers share the same connectivity but differ in spatial arrangement, resulting in similar yet distinguishable physical properties like optical activity, solubility, and melting points. Chemically, constitutional isomers exhibit different reactivity and reaction mechanisms, whereas diastereomers show differences in stereoselective reactions and interactions with chiral environments.
Real-World Examples and Applications
Constitutional isomers such as glucose and fructose differ in connectivity, influencing their use in food industry formulations and metabolic pathways. Diastereomers like cis- and trans-2-butene exhibit distinct physical properties critical in pharmaceuticals for drug design and efficacy. Understanding these differences enables chemists to tailor molecular structures for targeted applications in materials science and biochemistry.
Importance in Organic Synthesis and Drug Design
Constitutional isomers differ in connectivity of atoms, influencing physical and chemical properties, which is critical in organic synthesis for designing molecules with specific reactivity and stability. Diastereomers, stereoisomers that are not mirror images, exhibit distinct three-dimensional arrangements affecting pharmacodynamics and pharmacokinetics, essential for optimizing drug efficacy and minimizing side effects in drug design. Understanding these isomer types enables chemists to tailor molecular structures to achieve desired biological activity and synthetic accessibility.
Conclusion: Implications in Modern Chemistry
Constitutional isomers differ in the connectivity of atoms, leading to distinct chemical properties and reactivity, while diastereomers share the same connectivity but differ in spatial arrangement, impacting stereochemistry and biological activity. Understanding these differences is crucial for drug design, material science, and asymmetric synthesis, where precise molecular interactions dictate function and efficacy. Advances in spectroscopic techniques and computational chemistry further enhance the identification and utilization of these isomer types in modern chemical research and applications.
Constitutional isomer Infographic
 libterm.com
libterm.com