Lipophilic substances easily dissolve in fats and oils rather than water, making them crucial in fields like pharmacology and cosmetics for efficient delivery and absorption. Understanding the lipophilic properties of compounds helps optimize formulations and therapies tailored to your needs. Discover more about how lipophilicity impacts everyday products and scientific advancements in the rest of this article.
Table of Comparison
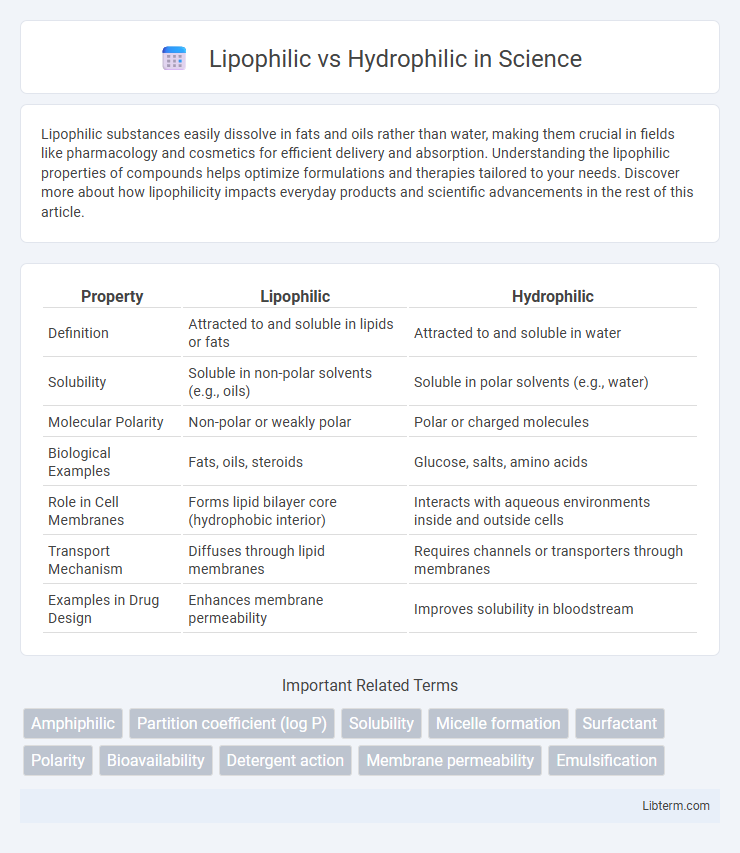
| Property | Lipophilic | Hydrophilic |
|---|---|---|
| Definition | Attracted to and soluble in lipids or fats | Attracted to and soluble in water |
| Solubility | Soluble in non-polar solvents (e.g., oils) | Soluble in polar solvents (e.g., water) |
| Molecular Polarity | Non-polar or weakly polar | Polar or charged molecules |
| Biological Examples | Fats, oils, steroids | Glucose, salts, amino acids |
| Role in Cell Membranes | Forms lipid bilayer core (hydrophobic interior) | Interacts with aqueous environments inside and outside cells |
| Transport Mechanism | Diffuses through lipid membranes | Requires channels or transporters through membranes |
| Examples in Drug Design | Enhances membrane permeability | Improves solubility in bloodstream |
Introduction to Lipophilic and Hydrophilic Properties
Lipophilic substances exhibit an affinity for lipid environments, dissolving readily in fats, oils, and nonpolar solvents due to their nonpolar molecular structure. Hydrophilic compounds, characterized by polar or charged groups, interact strongly with water molecules, enhancing solubility in aqueous environments. These contrasting properties influence the absorption, distribution, and bioavailability of compounds in biological systems and pharmaceutical formulations.
Defining Lipophilic Substances
Lipophilic substances are compounds that have an affinity for lipids and readily dissolve in fats, oils, and non-polar solvents due to their non-polar molecular structure. These substances typically exhibit low solubility in water and high solubility in organic solvents, influencing absorption and distribution in biological systems. Understanding the lipophilic nature of chemicals is crucial for drug design, environmental science, and material engineering, as it affects bioavailability and interaction with cell membranes.
Understanding Hydrophilic Compounds
Hydrophilic compounds possess polar molecules or ionic structures that allow them to readily dissolve in water through hydrogen bonding and electrostatic interactions. These substances are crucial in biological systems for functions like cellular transport, metabolism, and enzyme activity due to their affinity for aqueous environments. Understanding the molecular characteristics of hydrophilic compounds aids in drug formulation, biochemical analysis, and environmental science by predicting solubility and interaction with water-based mediums.
Key Differences: Lipophilic vs Hydrophilic
Lipophilic substances dissolve readily in nonpolar solvents such as oils and fats, whereas hydrophilic substances dissolve well in polar solvents like water. Lipophilic molecules tend to be nonpolar or slightly polar, facilitating their interaction with lipid membranes, while hydrophilic molecules possess polar groups or charges, enhancing solubility in aqueous environments. Key differences include solubility preferences, molecular polarity, and interactions with biological membranes impacting drug absorption and distribution.
Mechanisms of Lipophilicity and Hydrophilicity
Lipophilicity refers to the affinity of a molecule for lipid environments, driven by nonpolar interactions and the molecule's ability to dissolve in hydrophobic solvents due to its nonpolar or weakly polar nature. Hydrophilicity, on the other hand, involves strong interactions with water molecules facilitated by polar functional groups and hydrogen bonding capabilities, enabling dissolution in aqueous environments. The mechanisms underlying lipophilicity and hydrophilicity fundamentally influence compound solubility, membrane permeability, and pharmacokinetic profiles crucial in drug design and biochemical processes.
Biological Roles in Cellular Membranes
Lipophilic molecules, such as phospholipid tails, play a critical role in forming the hydrophobic core of cellular membranes, ensuring membrane integrity and selective permeability. Hydrophilic molecules, including polar head groups and membrane proteins, interact with the aqueous intracellular and extracellular environments, facilitating signal transduction and transport processes. The interplay between lipophilic and hydrophilic components maintains membrane fluidity and supports vital cellular functions like nutrient uptake and ion regulation.
Pharmaceutical Implications and Drug Design
Lipophilic drugs demonstrate enhanced membrane permeability, facilitating passive diffusion across lipid bilayers, which is crucial for oral bioavailability and crossing the blood-brain barrier. Hydrophilic compounds, conversely, often require transporter-mediated uptake or parenteral administration due to limited lipid solubility, impacting drug distribution and elimination profiles. Optimizing the balance between lipophilicity and hydrophilicity is essential in drug design to achieve targeted delivery, controlled release, and improved pharmacokinetic properties.
Environmental Impact and Solubility
Lipophilic substances easily dissolve in fats and oils, facilitating their accumulation in living organisms and potential bioaccumulation in ecosystems, which can lead to long-term environmental contamination. Hydrophilic compounds readily dissolve in water, enhancing their transport and dispersion in aquatic environments but also increasing the risk of widespread pollution in water bodies. Understanding the solubility properties of chemicals is crucial for predicting their environmental fate and implementing appropriate remediation strategies.
Practical Applications in Industry
Lipophilic compounds, soluble in fats and oils, are extensively used in pharmaceuticals for drug delivery systems targeting lipid-rich tissues, enhancing bioavailability and therapeutic efficacy. Hydrophilic substances, which dissolve readily in water, play crucial roles in industries like cosmetics and food processing, where moisture retention and solubility are essential for product formulation and stability. The contrasting solubility properties drive innovation in manufacturing emulsions, detergents, and coatings, optimizing performance based on the interaction with lipid or aqueous environments.
Choosing Lipophilic or Hydrophilic Agents
Choosing lipophilic or hydrophilic agents depends on the target environment and desired therapeutic effect. Lipophilic agents readily dissolve in fats and cell membranes, making them ideal for crossing biological barriers such as the blood-brain barrier or targeting lipid-rich tissues. Hydrophilic agents, designed to dissolve in aqueous solutions, are preferred for systemic circulation, extracellular spaces, and applications requiring rapid distribution in bodily fluids.
Lipophilic Infographic
 libterm.com
libterm.com