Flow cytometry is a powerful technique used to analyze the physical and chemical characteristics of cells or particles as they flow in a fluid stream through a beam of light. This method enables rapid multiparametric analysis, making it essential for immunophenotyping, cell sorting, and biomarker detection in research and clinical diagnostics. Explore the rest of the article to understand how flow cytometry can enhance your cell analysis workflows.
Table of Comparison
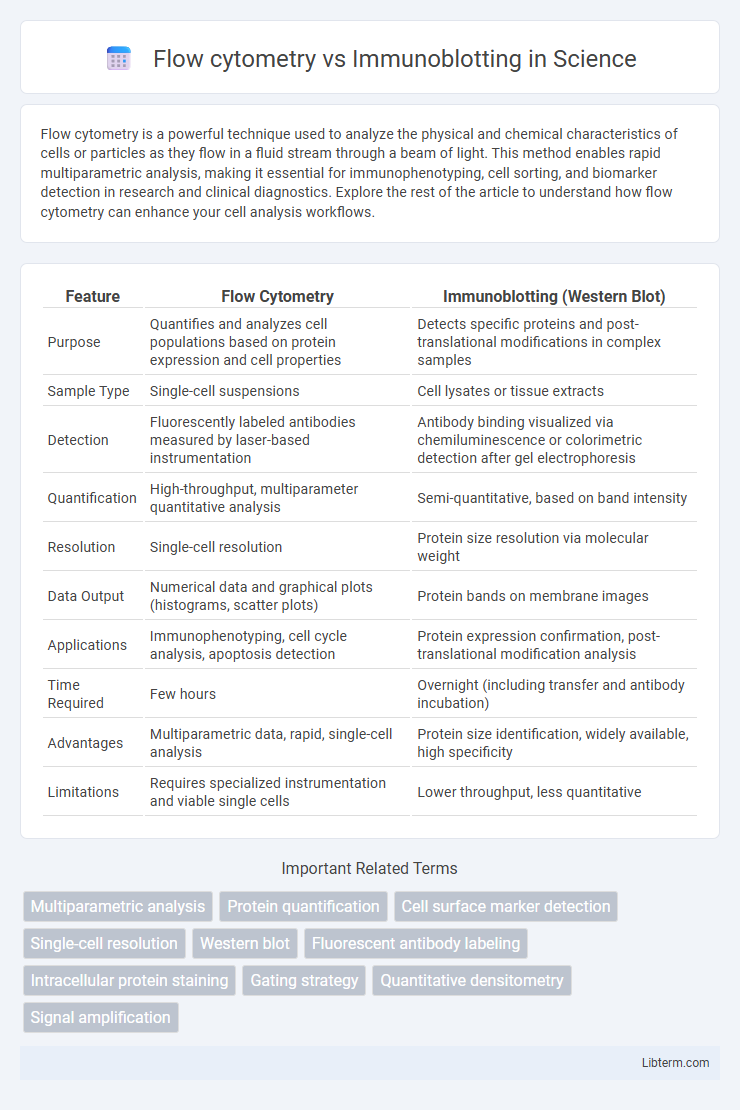
| Feature | Flow Cytometry | Immunoblotting (Western Blot) |
|---|---|---|
| Purpose | Quantifies and analyzes cell populations based on protein expression and cell properties | Detects specific proteins and post-translational modifications in complex samples |
| Sample Type | Single-cell suspensions | Cell lysates or tissue extracts |
| Detection | Fluorescently labeled antibodies measured by laser-based instrumentation | Antibody binding visualized via chemiluminescence or colorimetric detection after gel electrophoresis |
| Quantification | High-throughput, multiparameter quantitative analysis | Semi-quantitative, based on band intensity |
| Resolution | Single-cell resolution | Protein size resolution via molecular weight |
| Data Output | Numerical data and graphical plots (histograms, scatter plots) | Protein bands on membrane images |
| Applications | Immunophenotyping, cell cycle analysis, apoptosis detection | Protein expression confirmation, post-translational modification analysis |
| Time Required | Few hours | Overnight (including transfer and antibody incubation) |
| Advantages | Multiparametric data, rapid, single-cell analysis | Protein size identification, widely available, high specificity |
| Limitations | Requires specialized instrumentation and viable single cells | Lower throughput, less quantitative |
Overview of Flow Cytometry and Immunoblotting
Flow cytometry is a powerful technique that analyzes the physical and chemical characteristics of cells or particles by suspending them in a fluid stream and passing them through a laser, enabling rapid multiparametric analysis of thousands of cells per second. Immunoblotting, also known as Western blotting, detects specific proteins in a complex sample by separating proteins via gel electrophoresis, transferring them onto a membrane, and probing with antibodies for precise identification. Both methods are essential in cell biology and immunology, with flow cytometry providing quantitative data on cell populations and immunoblotting offering qualitative and semi-quantitative insights into protein expression and modifications.
Principles Behind Flow Cytometry
Flow cytometry operates on the principle of laser-based technology to analyze the physical and chemical characteristics of cells or particles as they flow in a fluid stream through a beam of light. It simultaneously measures multiple parameters, including cell size, granularity, and fluorescence intensity, by detecting light scatter and emitted fluorescence from dye-labeled antibodies. This enables rapid, quantitative analysis of heterogeneous cell populations at a single-cell level, providing detailed insights into cell phenotypes and function.
Fundamentals of Immunoblotting Techniques
Immunoblotting, also known as Western blotting, is a technique that detects specific proteins in a sample through gel electrophoresis, transfer onto a membrane, and antibody-based detection. This method relies on primary antibodies binding target proteins and secondary antibodies conjugated with enzymes for visualization, typically via chemiluminescence or colorimetric assays. Unlike flow cytometry, which analyzes physical and chemical characteristics of cells in suspension, immunoblotting provides detailed information on protein size and abundance, essential for studying protein expression and post-translational modifications.
Sample Preparation: Flow Cytometry vs Immunoblotting
Flow cytometry sample preparation involves creating a single-cell suspension, often requiring cell staining with fluorescently labeled antibodies to target specific surface or intracellular markers. Immunoblotting sample preparation typically includes protein extraction, quantification, and denaturation before gel electrophoresis and transfer to a membrane for antibody detection. While flow cytometry analyzes cells in their native state, immunoblotting provides a detailed assessment of protein expression and post-translational modifications.
Detection Capabilities and Specificity
Flow cytometry offers high detection capabilities by analyzing multiple parameters simultaneously in thousands of individual cells, enabling precise quantification of cell-surface and intracellular markers with single-cell resolution. Immunoblotting, or Western blotting, provides specific detection of proteins through antibody binding, allowing identification and characterization of protein size and post-translational modifications but lacks single-cell analysis. While flow cytometry excels in multiplexed, quantitative detection of protein expression patterns in heterogeneous cell populations, immunoblotting delivers qualitative and semi-quantitative data with high specificity for protein isoforms and modifications.
Quantitative vs Qualitative Data Output
Flow cytometry provides highly quantitative data by measuring fluorescence intensities on a cell-by-cell basis, enabling precise analysis of protein expression levels and cell population distributions. Immunoblotting, or Western blotting, primarily offers qualitative and semi-quantitative information by detecting specific protein presence and relative abundance through band intensity on membranes. While flow cytometry excels in quantifying multiple markers simultaneously within heterogeneous samples, immunoblotting is often used for validation and detailed protein characterization.
Multiplexing: Comparison of Analysis Capacity
Flow cytometry enables high-throughput multiplexing by simultaneously analyzing multiple fluorescently labeled antibodies on individual cells, allowing detailed characterization of cell populations and protein expression patterns. Immunoblotting, while useful for detecting specific proteins, has limited multiplexing capabilities due to the need for separate membranes or sequential probing for multiple targets. Consequently, flow cytometry offers superior multiplex analysis capacity for complex biological samples compared to immunoblotting.
Sensitivity and Limitations of Each Method
Flow cytometry offers high sensitivity in detecting and quantifying cell surface and intracellular proteins at a single-cell level, enabling multi-parameter analysis with rapid data acquisition. Immunoblotting, or Western blotting, provides high specificity for protein detection and identification but is less sensitive for low-abundance proteins and lacks single-cell resolution. Limitations of flow cytometry include complexity in sample preparation and potential signal overlap, while immunoblotting is time-consuming and offers only semi-quantitative results without cell population heterogeneity insights.
Common Applications in Research and Diagnostics
Flow cytometry enables rapid quantification and analysis of cell populations by measuring physical and chemical characteristics, widely used for immunophenotyping, cell cycle analysis, and detecting biomarkers in clinical diagnostics. Immunoblotting, or Western blotting, provides detailed protein identification and quantification, essential for studying protein expression, post-translational modifications, and validating antibody specificity in research. Both techniques complement each other in diagnostics, with flow cytometry offering high-throughput cell-based analysis and immunoblotting delivering precise protein profiling critical for disease diagnosis and monitoring.
Choosing the Right Technique for Your Study
Flow cytometry offers high-throughput single-cell analysis with quantitative multiparametric data, ideal for phenotyping and functional assays in heterogeneous populations. Immunoblotting provides qualitative and semi-quantitative detection of specific proteins, making it suited for confirming protein expression and post-translational modifications. Selecting the appropriate technique depends on study goals, sample complexity, and the need for quantitative versus qualitative data.
Flow cytometry Infographic
 libterm.com
libterm.com