Gamma decay involves the emission of high-energy photons from an excited nucleus, resulting in a lower energy state without changing its atomic number or mass. This process plays a crucial role in nuclear physics and radiation shielding due to the penetrating nature of gamma rays. Explore the article to understand how gamma decay impacts both natural phenomena and technological applications.
Table of Comparison
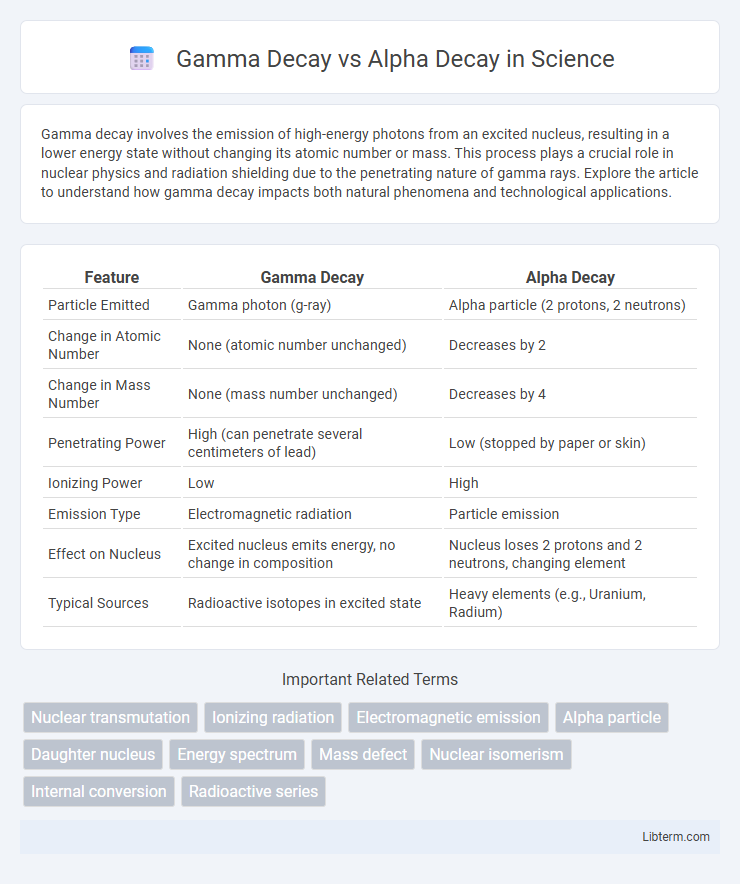
| Feature | Gamma Decay | Alpha Decay |
|---|---|---|
| Particle Emitted | Gamma photon (g-ray) | Alpha particle (2 protons, 2 neutrons) |
| Change in Atomic Number | None (atomic number unchanged) | Decreases by 2 |
| Change in Mass Number | None (mass number unchanged) | Decreases by 4 |
| Penetrating Power | High (can penetrate several centimeters of lead) | Low (stopped by paper or skin) |
| Ionizing Power | Low | High |
| Emission Type | Electromagnetic radiation | Particle emission |
| Effect on Nucleus | Excited nucleus emits energy, no change in composition | Nucleus loses 2 protons and 2 neutrons, changing element |
| Typical Sources | Radioactive isotopes in excited state | Heavy elements (e.g., Uranium, Radium) |
Introduction to Nuclear Decay
Gamma decay involves the emission of high-energy photons from an excited nucleus, resulting in a transition to a lower energy state without changing the atomic number or mass number. Alpha decay consists of the emission of an alpha particle, which is a helium nucleus containing two protons and two neutrons, causing the parent nucleus to decrease its atomic number by two and its mass number by four. Both processes are fundamental types of nuclear decay that help unstable nuclei achieve greater stability through different mechanisms of energy release.
What is Alpha Decay?
Alpha decay is a type of radioactive decay where an unstable nucleus emits an alpha particle consisting of two protons and two neutrons, resulting in a new nucleus with atomic number reduced by two and mass number reduced by four. This process decreases the original element's atomic mass and transforms it into a different element, often seen in heavy elements like uranium and radium. Unlike gamma decay, alpha decay involves the emission of particles rather than electromagnetic radiation.
What is Gamma Decay?
Gamma decay is a type of radioactive decay where an unstable atomic nucleus releases excess energy by emitting high-energy gamma photons. Unlike alpha decay, which emits helium nuclei, gamma decay involves no change in the number of protons or neutrons, resulting in no alteration to the element's atomic number or mass. Gamma rays possess extremely short wavelengths and high electromagnetic energy, making gamma decay a key process in nuclear transitions and radiation emissions.
Key Differences Between Alpha and Gamma Decay
Alpha decay involves the emission of a helium nucleus consisting of two protons and two neutrons, resulting in a decrease in atomic mass and atomic number of the parent nucleus. In contrast, gamma decay releases high-energy photons without altering the atomic number or mass, instead lowering the nucleus's energy state. Alpha decay causes significant nuclear transformation and changes in chemical identity, whereas gamma decay primarily involves energy emission without particle loss or elemental change.
Mechanisms of Alpha Decay
Alpha decay involves the emission of an alpha particle, consisting of two protons and two neutrons, from a heavy nucleus, resulting in the reduction of atomic number by two and mass number by four. This process occurs due to quantum tunneling, where the alpha particle penetrates the nuclear potential barrier despite insufficient classical energy to overcome it. In contrast, gamma decay emits high-energy photons without changing the atomic number or mass, occurring as the nucleus transitions from an excited to a lower energy state.
Mechanisms of Gamma Decay
Gamma decay involves the emission of high-energy photons from an excited nucleus returning to a lower energy state, without changing the number of protons or neutrons. This process occurs after alpha or beta decay leaves the daughter nucleus in an excited state, allowing it to release excess energy through gamma-ray emission. Unlike alpha decay, which ejects helium nuclei, gamma decay solely emits electromagnetic radiation and does not alter the atomic mass or atomic number.
Energy Emission in Alpha vs Gamma Decay
Alpha decay releases energy primarily through the emission of a helium nucleus, consisting of two protons and two neutrons, carrying substantial kinetic energy typically around 4 to 8 MeV. Gamma decay involves the release of electromagnetic energy in the form of high-energy photons, with energies ranging from keV to several MeV, depending on the nuclear transition. The energy emitted in alpha decay is generally higher per particle compared to the discrete photon energies in gamma decay, reflecting differences in decay mechanisms and nuclear structure.
Biological and Environmental Effects
Gamma decay emits high-energy photons that penetrate tissues deeply, potentially causing DNA damage and increasing cancer risk in living organisms. Alpha decay releases alpha particles with low penetration power, causing significant localized damage to biological cells if ingested or inhaled, as seen in radon exposure linked to lung cancer. Environmentally, gamma radiation affects a broader area due to its penetrating ability, while alpha emitters primarily contaminate surfaces or materials, posing hazards through ingestion or inhalation pathways.
Detection Methods for Alpha and Gamma Particles
Alpha particles are commonly detected using scintillation counters and semiconductor detectors, which efficiently measure their high ionization and short penetration depth. Gamma radiation requires more sensitive detection methods such as high-purity germanium detectors and sodium iodide scintillators, capable of measuring the high-energy photons and their deeper penetration. Both detection techniques rely on the particles' distinct interactions with materials, enabling precise identification and differentiation between gamma and alpha radiation.
Applications in Science and Medicine
Gamma decay is extensively utilized in medical imaging techniques such as PET scans and cancer radiotherapy due to its high penetration power and ability to target deep tissues without causing significant damage to surrounding areas. Alpha decay finds applications in targeted alpha therapy (TAT) for cancer treatment, where alpha particles deliver lethal damage to cancer cells while minimizing harm to healthy tissue because of their short range and high ionization capability. Both decay processes are critical in scientific research for radiometric dating and tracing biological processes, with gamma emissions providing precise detection and alpha emissions enabling localized treatment effects.
Gamma Decay Infographic
 libterm.com
libterm.com