Reversible clothing offers versatile style options by allowing you to wear the garment inside out, effectively doubling your wardrobe choices. This innovative design combines practicality with fashion, making it ideal for travelers and minimalists seeking functionality without sacrificing appearance. Discover how reversible items can transform your outfit strategy by exploring the rest of this article.
Table of Comparison
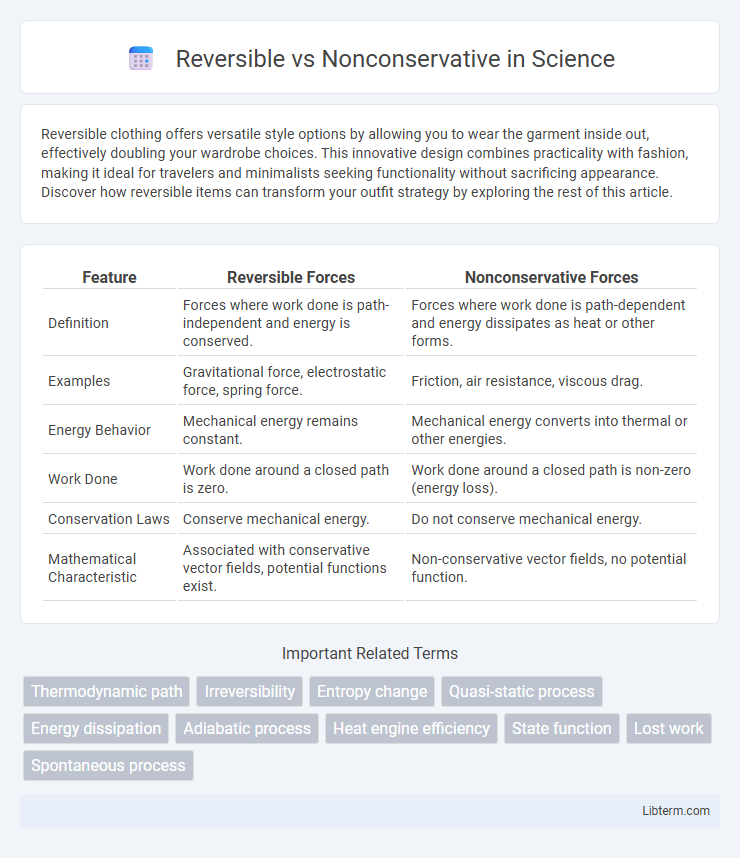
| Feature | Reversible Forces | Nonconservative Forces |
|---|---|---|
| Definition | Forces where work done is path-independent and energy is conserved. | Forces where work done is path-dependent and energy dissipates as heat or other forms. |
| Examples | Gravitational force, electrostatic force, spring force. | Friction, air resistance, viscous drag. |
| Energy Behavior | Mechanical energy remains constant. | Mechanical energy converts into thermal or other energies. |
| Work Done | Work done around a closed path is zero. | Work done around a closed path is non-zero (energy loss). |
| Conservation Laws | Conserve mechanical energy. | Do not conserve mechanical energy. |
| Mathematical Characteristic | Associated with conservative vector fields, potential functions exist. | Non-conservative vector fields, no potential function. |
Introduction to Reversible and Nonconservative Processes
Reversible processes are idealized thermodynamic changes that occur infinitely slowly, allowing the system to remain in equilibrium with its surroundings and enabling the process to be reversed without net entropy change. Nonconservative processes involve irreversible phenomena such as friction, turbulence, or unrestrained expansion, leading to entropy production and energy dissipation within the system. Understanding these distinctions is crucial for analyzing energy efficiency and entropy management in physical and engineering systems.
Defining Reversible Processes
Reversible processes are idealized thermodynamic changes that occur infinitely slowly, allowing the system to remain in thermodynamic equilibrium throughout. These processes conserve entropy, meaning no entropy is generated within the system or surroundings, ensuring maximum efficiency. In contrast, nonconservative or irreversible processes generate entropy due to factors like friction, unrestrained expansion, or spontaneous heat transfer, making them unable to return both system and surroundings to the original state without net changes.
Understanding Nonconservative Processes
Nonconservative processes involve energy transformations where mechanical energy is not conserved due to forces like friction, air resistance, or inelastic collisions converting mechanical energy into thermal energy. These processes result in a net loss of usable mechanical energy, distinguishing them from reversible, conservative systems where energy remains within the mechanical domain. Understanding nonconservative forces is crucial for accurately analyzing real-world systems and predicting energy dissipation.
Key Differences Between Reversible and Nonconservative Processes
Reversible processes occur without entropy generation and can return both the system and surroundings to their original states, making them idealized and highly efficient. Nonconservative processes involve energy dissipation, such as friction or unrestrained expansion, causing irreversibility and an increase in entropy. The key difference lies in the reversibility criterion, where reversible processes maintain thermodynamic equilibrium throughout, whereas nonconservative processes drive the system away from equilibrium due to irreversible energy losses.
Thermodynamic Principles Behind Reversibility
Reversible processes in thermodynamics are idealized transformations that occur infinitely slowly, maintaining equilibrium and maximizing efficiency by minimizing entropy generation. Nonconservative processes involve dissipation of energy, creating irreversibility and increasing the total entropy of the system and surroundings. The Second Law of Thermodynamics fundamentally distinguishes reversible processes as those with zero net entropy change, while nonconservative processes contribute to the irreversibility and degradation of energy quality.
Energy Dissipation in Nonconservative Systems
Nonconservative systems dissipate energy through mechanisms such as friction, air resistance, and inelastic deformations, causing a loss of mechanical energy converted into heat or other forms. Unlike reversible systems where total energy remains constant, energy in nonconservative systems decreases over time, leading to irreversible changes in system behavior. Understanding energy dissipation in nonconservative forces is crucial for accurately modeling real-world mechanical and thermodynamic processes.
Real-world Examples of Reversible Processes
Reversible processes occur in idealized systems where changes happen infinitely slowly, such as the slow compression of a gas in a piston or the melting of ice at its exact melting point. These processes can be reversed without increasing entropy, making them an important concept in thermodynamics for understanding maximum efficiency in engines and refrigerators. In contrast, nonconservative processes involve irreversible changes common in everyday phenomena like friction, spontaneous heat transfer, and chemical reactions, where energy is dissipated as heat and entropy increases.
Common Instances of Nonconservative Processes
Nonconservative processes commonly occur in frictional force interactions, where mechanical energy dissipates as heat, and in air resistance effects slowing down moving objects, causing energy loss from the system. Another typical example includes inelastic collisions, where kinetic energy is not conserved due to deformation and internal energy changes. These instances contrast reversible processes by involving irreversible energy transformations and entropy increases.
Practical Implications in Engineering and Science
Reversible processes, characterized by minimal entropy generation, enable engineers to design highly efficient thermodynamic systems such as ideal heat engines and refrigeration cycles, maximizing work output and minimizing energy losses. Nonconservative processes involve irreversible effects like friction, turbulence, and unrestrained expansion, which increase entropy and reduce system efficiency, necessitating compensatory design measures in practical engineering applications. Understanding the distinction guides the optimization of energy conversion devices and informs the development of sustainable technologies in mechanical, chemical, and aerospace engineering.
Comparing Efficiency and Entropy Generation
Reversible processes exhibit maximum thermodynamic efficiency by minimizing entropy generation, maintaining equilibrium states throughout energy transfer. Nonconservative, or irreversible processes, generate additional entropy due to friction, unrestrained expansion, and heat transfer with finite temperature differences, thus reducing overall system efficiency. The comparison highlights that reversible processes approach ideal efficiency limits, while nonconservative processes inherently suffer performance losses due to entropy increase.
Reversible Infographic
 libterm.com
libterm.com