Orthoclase and Albite are essential feldspar minerals commonly found in igneous and metamorphic rocks, with Orthoclase being a potassium-rich feldspar and Albite a sodium-rich counterpart. Their distinct crystal structures and chemical compositions influence the texture and durability of granites and other rock types, making them vital for geological studies and industrial applications. Explore this article to understand how these minerals impact your geological knowledge and their practical uses in various fields.
Table of Comparison
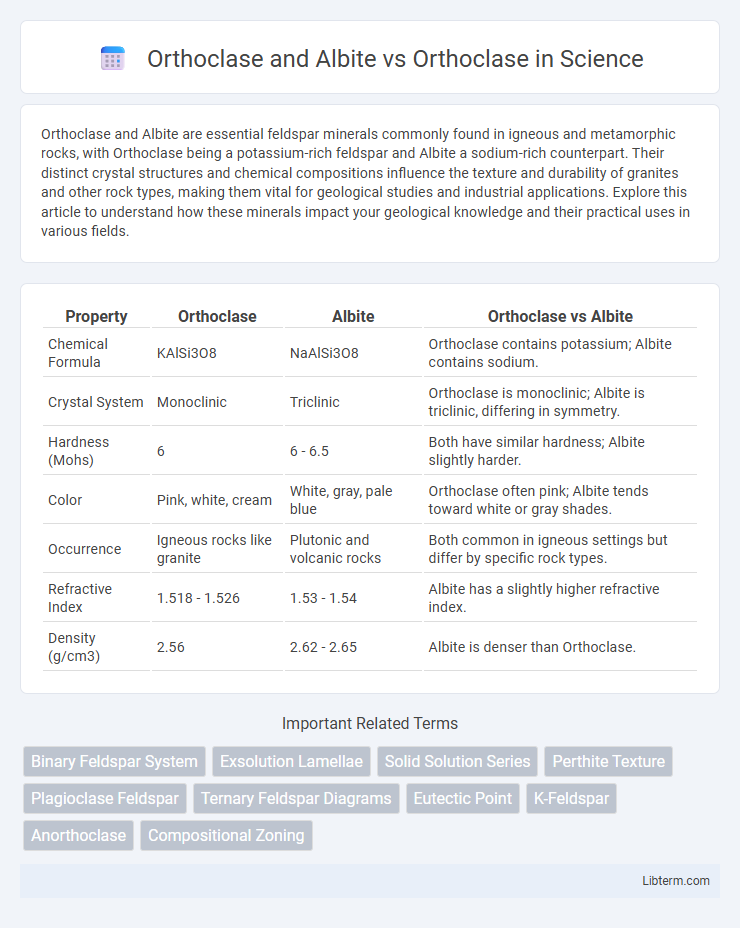
| Property | Orthoclase | Albite | Orthoclase vs Albite |
|---|---|---|---|
| Chemical Formula | KAlSi3O8 | NaAlSi3O8 | Orthoclase contains potassium; Albite contains sodium. |
| Crystal System | Monoclinic | Triclinic | Orthoclase is monoclinic; Albite is triclinic, differing in symmetry. |
| Hardness (Mohs) | 6 | 6 - 6.5 | Both have similar hardness; Albite slightly harder. |
| Color | Pink, white, cream | White, gray, pale blue | Orthoclase often pink; Albite tends toward white or gray shades. |
| Occurrence | Igneous rocks like granite | Plutonic and volcanic rocks | Both common in igneous settings but differ by specific rock types. |
| Refractive Index | 1.518 - 1.526 | 1.53 - 1.54 | Albite has a slightly higher refractive index. |
| Density (g/cm3) | 2.56 | 2.62 - 2.65 | Albite is denser than Orthoclase. |
Introduction to Orthoclase and Albite
Orthoclase and albite are both feldspar minerals commonly found in igneous and metamorphic rocks, playing crucial roles in rock formation and mineralogy. Orthoclase is a potassium-rich feldspar characterized by its crystal structure and pink to white coloration, while albite is a sodium-rich plagioclase feldspar, typically white to gray. Together, orthoclase and albite form part of the feldspar group, influencing the physical and chemical properties of granite and other silicate rocks.
Chemical Composition: Orthoclase vs Albite
Orthoclase is a potassium-rich feldspar with the chemical formula KAlSi3O8, while albite is a sodium-rich feldspar represented by NaAlSi3O8. Both minerals belong to the feldspar group but differ in the dominant alkali metal, which affects their physical and chemical properties. The substitution of potassium in orthoclase by sodium in albite results in variations in crystal structure and melting points, critical for petrological classification.
Crystal Structure Differences
Orthoclase and albite, both feldspar minerals, differ significantly in their crystal structures, with orthoclase exhibiting a monoclinic system characterized by a single twofold axis of symmetry, while albite crystallizes in a triclinic system with lower symmetry. The potassium ion in orthoclase integrates into a framework of silicon-oxygen tetrahedra, forming a stable three-dimensional network, whereas albite contains sodium ions substituting potassium, resulting in subtle distortions in the tetrahedral arrangement. These structural variations impact physical properties such as cleavage and refractive indices, with orthoclase typically showing more perfect cleavage due to its simpler symmetry compared to albite's more complex triclinic lattice.
Physical Properties Comparison
Orthoclase and Albite both belong to the feldspar group but exhibit distinct physical properties; Orthoclase typically has a Mohs hardness of 6, whereas Albite is slightly softer at 6 to 6.5. Orthoclase displays a monoclinic crystal system with a creamy white or pinkish color, while Albite crystallizes in the triclinic system, often appearing white or pale gray. Both minerals have similar specific gravities around 2.56-2.60, but Albite's cleavage is more perfect with two distinct directions compared to Orthoclase's less perfect cleavage.
Occurrence and Formation
Orthoclase commonly forms in granitic and pegmatitic environments, while albite frequently occurs in both igneous and metamorphic rocks, often within plagioclase feldspar series. Orthoclase and albite together crystallize from felsic magmas during slow cooling, resulting in intergrowths like perthite. Pure orthoclase typically forms in high-temperature, potassium-rich environments, whereas albite forms under a wider range of conditions, including low-temperature hydrothermal alteration.
Industrial and Practical Uses
Orthoclase and Albite, both feldspar minerals, are widely used in the ceramics and glass industries due to their high alumina and alkali content, which improve the strength and durability of products. Orthoclase is preferred for manufacturing porcelain, glass, and enamel, while the combination of Orthoclase and Albite enhances the melting behavior and thermal stability in industrial applications. Their use in electronics, such as insulators and substrates, leverages their electrical properties and chemical resistance, making these minerals essential in practical industry contexts.
Optical Properties: Orthoclase and Albite
Orthoclase and Albite exhibit distinct optical properties valuable in mineral identification; Orthoclase typically shows low birefringence and a first-order gray to white interference color under polarized light, while Albite displays higher birefringence with vibrant interference colors ranging from first-order yellow to second-order blue. Both minerals are part of the feldspar group but differ in refractive indices, with Orthoclase averaging at 1.518 to 1.530 and Albite ranging from 1.528 to 1.543, influencing how light interacts with their crystal structures. These variations in optical behavior assist geologists in differentiating between Orthoclase and Albite in petrographic microscopy.
Geological Significance
Orthoclase and Albite together form a crucial component of the feldspar group, significantly influencing the mineralogical composition of igneous and metamorphic rocks. Orthoclase, a potassium-rich feldspar, often marks the presence of granitic and syenitic formations, while Albite, a sodium-rich feldspar, is predominant in plagioclase series contributing to the understanding of magmatic differentiation and crystallization processes. Their coexistence reveals vital information about rock formation temperatures and tectonic settings, aiding geologists in reconstructing Earth's crustal evolution.
Orthoclase vs Orthoclase: Twin Formation and Variations
Orthoclase exhibits characteristic twin formations known as Carlsbad and Manebach twins, which arise from its monoclinic crystal structure and play a key role in its identification. Albite, part of the plagioclase feldspar group, shows albite twinning, differing significantly from orthoclase twins and affecting its optical properties. Variations in orthoclase twins result from differences in growth conditions and composition, influencing its mechanical behavior and use in geological studies.
Summary: Key Distinctions and Similarities
Orthoclase and Albite are both feldspar minerals, fundamental components of the Earth's crust, distinguished primarily by their chemical compositions--Orthoclase is a potassium-rich feldspar (KAlSi3O8) while Albite is sodium-rich (NaAlSi3O8). Both minerals share similar crystal structures classified under the monoclinic system and exhibit comparable physical properties such as hardness and cleavage. Their presence in igneous, metamorphic, and sedimentary rocks underscores their geological significance and the continuous solid solution series forming the plagioclase feldspar group.
Orthoclase and Albite Infographic
 libterm.com
libterm.com