Photolytic processes involve the use of light energy to break chemical bonds, enabling the transformation of substances in environmental and industrial applications. These reactions are crucial in areas such as water purification, air quality improvement, and the degradation of pollutants. Explore the article to understand how photolytic technologies can enhance Your environmental solutions.
Table of Comparison
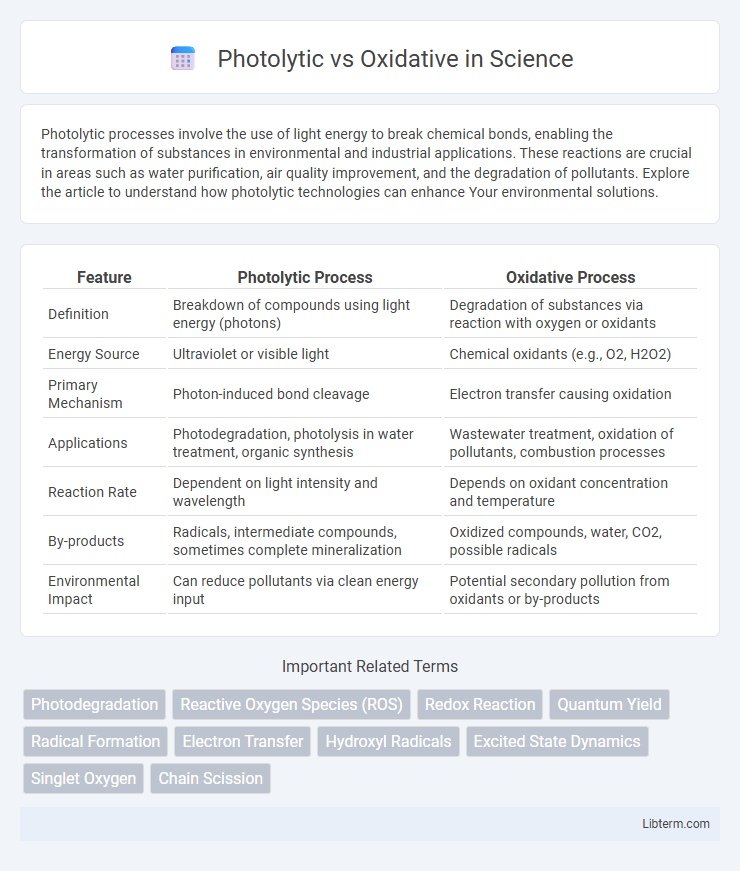
| Feature | Photolytic Process | Oxidative Process |
|---|---|---|
| Definition | Breakdown of compounds using light energy (photons) | Degradation of substances via reaction with oxygen or oxidants |
| Energy Source | Ultraviolet or visible light | Chemical oxidants (e.g., O2, H2O2) |
| Primary Mechanism | Photon-induced bond cleavage | Electron transfer causing oxidation |
| Applications | Photodegradation, photolysis in water treatment, organic synthesis | Wastewater treatment, oxidation of pollutants, combustion processes |
| Reaction Rate | Dependent on light intensity and wavelength | Depends on oxidant concentration and temperature |
| By-products | Radicals, intermediate compounds, sometimes complete mineralization | Oxidized compounds, water, CO2, possible radicals |
| Environmental Impact | Can reduce pollutants via clean energy input | Potential secondary pollution from oxidants or by-products |
Introduction to Photolytic and Oxidative Processes
Photolytic processes involve the absorption of light energy to break chemical bonds, initiating reactions such as photodegradation and photopolymerization. Oxidative processes rely on the transfer of electrons, leading to the formation of reactive oxygen species that drive oxidation reactions in environmental and biological systems. Understanding the mechanisms of photolytic and oxidative pathways is crucial for applications in pollution control, material science, and biochemical regulation.
Definition and Basic Principles
Photolytic processes involve the breaking down of chemical compounds through the absorption of light energy, typically ultraviolet (UV) radiation, resulting in molecular dissociation and the generation of reactive species. Oxidative processes rely on chemical reactions with oxygen or other oxidizing agents, leading to the transformation or degradation of substances through electron transfer and the formation of oxidized products. Both mechanisms are fundamental in environmental chemistry for pollutant degradation, but photolysis is driven by photon energy while oxidation is governed by redox reactions.
Mechanisms of Photolytic Reactions
Photolytic reactions involve the absorption of light energy causing bond cleavage and the formation of reactive radicals through homolytic dissociation. This process typically initiates when photons match the energy required to excite electrons to higher energy states, leading to molecular breakdown and subsequent reactions. Photolytic mechanisms differ from oxidative pathways by relying primarily on direct photonic energy rather than electron transfer from oxidizing agents.
Mechanisms of Oxidative Reactions
Oxidative reactions primarily involve the transfer of electrons leading to the formation of reactive oxygen species (ROS) such as hydrogen peroxide, superoxide anion, and hydroxyl radicals. These ROS initiate a cascade of molecular transformations by attacking organic substrates, causing bond cleavage, and generating intermediate radicals. Enzymatic systems like cytochrome P450 and peroxidases facilitate these electron transfer processes, driving oxidation through mechanisms distinct from photolytic cleavage.
Key Differences Between Photolytic and Oxidative Methods
Photolytic methods utilize light energy, primarily UV radiation, to break chemical bonds and induce reactions, favoring processes like photodegradation and photolysis. Oxidative methods rely on oxidizing agents, such as ozone, hydrogen peroxide, or oxygen, to chemically alter or decompose contaminants through electron transfer mechanisms. The key difference lies in the energy source--photolytic processes depend on photon energy, while oxidative processes involve chemical oxidation reactions without direct light involvement.
Advantages of Photolytic Processes
Photolytic processes utilize light energy to break down contaminants efficiently, offering precise control over reaction pathways compared to oxidative methods. These processes generate fewer harmful byproducts and exhibit higher selectivity in degrading specific pollutants such as organic compounds and pathogens. Enhanced energy efficiency and the ability to operate under milder conditions further emphasize the environmental and operational advantages of photolytic techniques in advanced water and air treatment applications.
Benefits of Oxidative Techniques
Oxidative techniques offer significant benefits in water and air purification by effectively breaking down organic contaminants into harmless byproducts, ensuring higher levels of detoxification. These methods, such as advanced oxidation processes (AOPs), enhance pollutant degradation through the generation of reactive oxygen species, leading to improved treatment efficiency compared to photolytic techniques alone. Enhanced mineralization and reduced formation of toxic intermediates further position oxidative treatments as superior solutions in environmental remediation applications.
Limitations and Challenges of Each Approach
Photolytic methods face limitations such as inefficient light penetration in turbid or colored solutions, restricting their effectiveness to transparent media. Oxidative processes encounter challenges including the formation of potentially harmful byproducts and the requirement for precise control of reaction conditions to avoid incomplete oxidation. Both approaches demand significant energy input, yet photolytic techniques often require expensive UV light sources while oxidative methods rely heavily on chemical reagents, impacting overall scalability and cost-efficiency.
Applications in Environmental and Industrial Fields
Photolytic processes harness light energy to break down pollutants, making them effective in water purification and air treatment by degrading hazardous organic compounds. Oxidative methods, employing agents like ozone or hydrogen peroxide, are widely applied in industrial wastewater treatment and soil remediation due to their strong oxidizing capabilities that mineralize contaminants. Both techniques are integral in environmental monitoring and industrial pollution control, with photolytic systems favored for energy efficiency and oxidative processes valued for broad-spectrum contaminant removal.
Future Trends and Innovations
Emerging innovations in photolytic and oxidative processes emphasize enhancing energy efficiency and selectivity through advanced catalyst development and integrated reactor designs. Future trends include leveraging solar-driven photolytic systems combined with nanomaterial-based oxidative catalysts to optimize reaction yields and reduce environmental impact. Research in hybrid photolytic-oxidative technologies aims to create sustainable, scalable solutions for chemical synthesis and pollution control industries.
Photolytic Infographic
 libterm.com
libterm.com