Nonpolar molecules have an even distribution of electrical charge, resulting in no permanent dipole moment and often making them hydrophobic. These molecules play crucial roles in biological processes and industrial applications due to their unique chemical properties. Explore the rest of the article to understand how nonpolar substances interact and influence your environment.
Table of Comparison
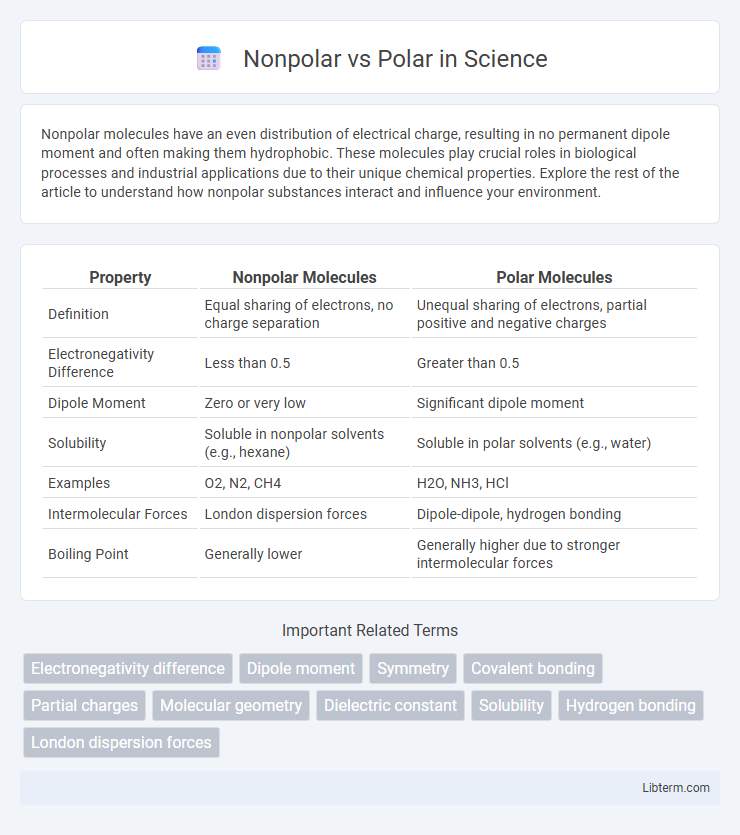
| Property | Nonpolar Molecules | Polar Molecules |
|---|---|---|
| Definition | Equal sharing of electrons, no charge separation | Unequal sharing of electrons, partial positive and negative charges |
| Electronegativity Difference | Less than 0.5 | Greater than 0.5 |
| Dipole Moment | Zero or very low | Significant dipole moment |
| Solubility | Soluble in nonpolar solvents (e.g., hexane) | Soluble in polar solvents (e.g., water) |
| Examples | O2, N2, CH4 | H2O, NH3, HCl |
| Intermolecular Forces | London dispersion forces | Dipole-dipole, hydrogen bonding |
| Boiling Point | Generally lower | Generally higher due to stronger intermolecular forces |
Introduction to Polarity: Nonpolar vs Polar
Molecules exhibit polarity based on the distribution of electron density, classifying them as nonpolar or polar. Nonpolar molecules have an even electron distribution resulting in no permanent dipole moment, commonly found in diatomic molecules like O2 and nonpolar covalent bonds. Polar molecules contain unequal electron sharing, creating partial positive and negative charges, exemplified by water (H2O) with its bent molecular geometry leading to a net dipole moment.
Understanding Molecular Polarity
Molecular polarity arises from the difference in electronegativity between atoms and the asymmetrical arrangement of bonds within a molecule. Nonpolar molecules have an even distribution of electron density, resulting in no permanent dipole moment, while polar molecules exhibit regions of partial positive and negative charges due to uneven electron sharing. Understanding molecular polarity is crucial for predicting solubility, intermolecular interactions, and chemical reactivity in various substances.
Key Differences Between Nonpolar and Polar Molecules
Nonpolar molecules have an equal distribution of electron density resulting in no permanent dipole moment, whereas polar molecules possess an uneven distribution of electrons, creating partial positive and negative charges. Nonpolar molecules typically consist of atoms with similar electronegativities or symmetric geometries, while polar molecules contain atoms with significant electronegativity differences and asymmetrical shapes. These key differences influence solubility, boiling points, and molecular interactions in chemical and biological systems.
How Electronegativity Influences Polarity
Electronegativity differences between atoms in a molecule determine its polarity, where significant differences create polar bonds due to unequal electron sharing, while small or no differences lead to nonpolar bonds with equal electron distribution. Molecules with polar bonds and an asymmetric shape exhibit a net dipole moment, making the entire molecule polar, whereas symmetrical molecules with polar bonds may be nonpolar overall due to balanced dipoles. Understanding electronegativity values, such as fluorine's 3.98 compared to hydrogen's 2.20, helps predict bond polarity and molecular behavior in solvents and reactions.
Examples of Nonpolar Molecules
Nonpolar molecules such as oxygen (O2), nitrogen (N2), and methane (CH4) exhibit an even distribution of electrical charge, resulting in no permanent dipole moment. These molecules typically consist of atoms with similar electronegativities or symmetric arrangements that cancel out bond dipoles. Examples like carbon dioxide (CO2) also highlight linear structures with polar bonds whose dipoles balance, maintaining overall nonpolarity.
Examples of Polar Molecules
Water (H2O) is a classic example of a polar molecule due to its bent shape and the electronegativity difference between hydrogen and oxygen atoms, resulting in a partial positive charge on hydrogen and a partial negative charge on oxygen. Other polar molecules include ammonia (NH3), which has a trigonal pyramidal shape causing an uneven distribution of electron density, and hydrogen chloride (HCl), where the significant electronegativity difference between hydrogen and chlorine atoms creates a dipole moment. These molecules exhibit polarity because of their molecular geometry and the unequal sharing of electrons, leading to distinct positive and negative poles.
Physical Properties: Comparing Nonpolar and Polar Substances
Nonpolar substances exhibit low boiling points and poor solubility in water due to weak van der Waals forces, resulting in high volatility and low polarity. Polar substances demonstrate higher boiling points and greater solubility in water because of strong dipole-dipole interactions and hydrogen bonding. These differences in intermolecular forces directly influence melting points, evaporation rates, and solvent compatibility in chemical and physical applications.
Solubility: Nonpolar vs Polar Compounds
Nonpolar compounds dissolve readily in nonpolar solvents due to similar intermolecular forces, following the principle "like dissolves like," whereas polar compounds are more soluble in polar solvents because of their ability to form hydrogen bonds and dipole-dipole interactions. Water, a highly polar solvent, efficiently dissolves polar substances such as salts and sugars but poorly dissolves nonpolar substances like oils and fats. Solubility differences between nonpolar and polar compounds significantly impact processes in chemistry, biology, and industrial applications involving mixtures and extractions.
Real-World Applications of Polarity
Nonpolar molecules such as oils and fats are essential in industrial lubricants and waterproof coatings due to their hydrophobic properties, preventing water interaction and corrosion. Polar substances like water and alcohols excel as solvents in pharmaceuticals and cleaning agents, dissolving ionic compounds and facilitating chemical reactions. Understanding molecular polarity enables innovations in drug formulation, environmental remediation, and material science by optimizing solubility and intermolecular interactions.
Summary and Importance of Molecular Polarity
Molecular polarity determines how molecules interact with each other and influences properties such as solubility, boiling points, and reactivity. Nonpolar molecules have an even distribution of charge, resulting in minimal electrical attraction, while polar molecules exhibit partial positive and negative charges due to uneven electron sharing. Understanding molecular polarity is crucial in fields like chemistry, biology, and materials science for predicting molecular behavior and designing compounds with desired characteristics.
Nonpolar Infographic
 libterm.com
libterm.com