Dynorphin is a naturally occurring opioid peptide in the brain that plays a crucial role in regulating pain, stress, and emotional responses by binding to kappa opioid receptors. Its unique mechanism helps modulate neurochemical pathways, influencing mood and behavior, which makes it a significant area of study for understanding addiction and mental health disorders. Explore the rest of this article to learn how dynorphin impacts your brain function and its potential therapeutic applications.
Table of Comparison
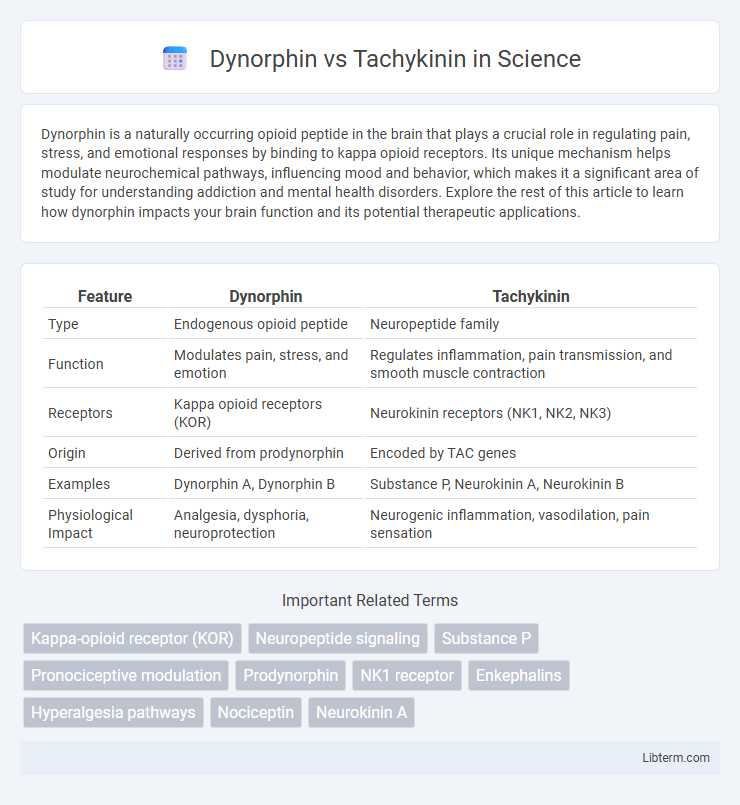
| Feature | Dynorphin | Tachykinin |
|---|---|---|
| Type | Endogenous opioid peptide | Neuropeptide family |
| Function | Modulates pain, stress, and emotion | Regulates inflammation, pain transmission, and smooth muscle contraction |
| Receptors | Kappa opioid receptors (KOR) | Neurokinin receptors (NK1, NK2, NK3) |
| Origin | Derived from prodynorphin | Encoded by TAC genes |
| Examples | Dynorphin A, Dynorphin B | Substance P, Neurokinin A, Neurokinin B |
| Physiological Impact | Analgesia, dysphoria, neuroprotection | Neurogenic inflammation, vasodilation, pain sensation |
Introduction to Dynorphin and Tachykinin
Dynorphin is an endogenous opioid peptide that primarily binds to kappa-opioid receptors, playing a crucial role in modulating pain, stress response, and emotional regulation. Tachykinins, including substance P and neurokinin A, are a family of neuropeptides that activate neurokinin receptors involved in neurotransmission, inflammation, and smooth muscle contraction. Both dynorphin and tachykinins contribute significantly to neurochemical signaling but differ in receptor targets and physiological effects.
Molecular Structure and Biosynthesis
Dynorphins are opioid peptides derived from the precursor protein prodynorphin, characterized by the presence of a unique tyrosine-containing sequence critical for binding to kappa-opioid receptors. Tachykinins, such as substance P, are neuropeptides synthesized from preprotachykinin genes, featuring a conserved C-terminal sequence (Phe-X-Gly-Leu-Met-NH2) essential for receptor interaction. The biosynthesis of dynorphins involves proteolytic cleavage and post-translational modifications within secretory vesicles, while tachykinins arise from alternative splicing and enzymatic processing of their larger precursor proteins in neuronal and endocrine cells.
Receptor Binding and Signal Transduction
Dynorphins primarily bind to kappa-opioid receptors (KORs), triggering G-protein-coupled receptor (GPCR) pathways that inhibit adenylate cyclase activity, reduce cAMP levels, and modulate ion channel conductance to produce analgesic and dysphoric effects. Tachykinins, such as substance P, interact predominantly with neurokinin receptors (NK1, NK2, NK3), activating phospholipase C via Gq/11 proteins, leading to increased intracellular calcium and protein kinase C stimulation, which facilitates neurogenic inflammation and pain transmission. These distinct receptor binding profiles and downstream signaling mechanisms underscore the divergent physiological roles of dynorphins and tachykinins in neurochemical communication.
Distribution in the Central Nervous System
Dynorphin peptides are predominantly distributed within the limbic system, including the hippocampus, amygdala, and hypothalamus, where they modulate pain, stress response, and emotional behavior. Tachykinins, such as substance P, exhibit widespread distribution throughout the central nervous system, especially in the spinal cord, brainstem, and basal ganglia, contributing to nociception and neural communication. The contrasting localization highlights dynorphin's role in neuroendocrine regulation versus tachykinins' primary function in sensory transmission and motor control.
Physiological Functions and Roles
Dynorphins primarily modulate pain perception, stress response, and mood regulation through their interaction with kappa opioid receptors, influencing analgesia and neuroendocrine functions. Tachykinins, such as substance P, play critical roles in neurogenic inflammation, pain transmission, and smooth muscle contraction by activating neurokinin receptors in the peripheral and central nervous systems. Both peptide families contribute to complex signaling pathways affecting cardiovascular, respiratory, and gastrointestinal physiology, yet dynorphins predominantly regulate opioid-related pathways while tachykinins are key mediators in excitatory neurotransmission and inflammatory responses.
Involvement in Pain Modulation
Dynorphin, an endogenous opioid peptide, plays a crucial role in pain modulation by binding to kappa opioid receptors (KOR), leading to analgesic effects and the inhibition of pain signals in the central nervous system. Tachykinins, including substance P and neurokinin A, bind to neurokinin receptors (NK1, NK2) and primarily facilitate pain transmission and inflammatory responses, enhancing nociceptive signaling. The opposing actions of dynorphins and tachykinins regulate the balance between pain inhibition and facilitation, significantly influencing chronic pain and neuropathic pain states.
Impact on Stress and Emotional Responses
Dynorphins, a class of opioid peptides, primarily modulate stress by activating kappa opioid receptors, resulting in dysphoric and anxiety-like effects that influence emotional regulation. Tachykinins, including substance P, interact with neurokinin receptors to enhance stress responses and promote anxiety through the activation of limbic brain regions. The opposing roles of dynorphins and tachykinins in stress and emotional responses highlight their significance as targets for therapeutic interventions in mood disorders.
Dynorphin vs Tachykinin: Key Differences
Dynorphins are opioid peptides primarily involved in modulating pain, stress, and emotional responses by binding to kappa-opioid receptors, whereas tachykinins, such as substance P, function mainly as neuropeptides influencing inflammation, pain transmission, and smooth muscle contraction through neurokinin receptors. Dynorphin peptides typically regulate analgesia and dysphoria, contrasting with tachykinins' roles in promoting neurogenic inflammation and vasodilation. The key difference lies in their receptor specificity and physiological impacts, with dynorphins acting within the opioid system and tachykinins operating primarily through neurokinin receptors.
Clinical Implications and Therapeutic Potential
Dynorphins, as opioid peptides primarily involved in modulating pain and stress responses, show promising therapeutic potential in treating chronic pain and addiction by targeting kappa-opioid receptors to reduce dependency and inflammation. Tachykinins, including substance P, play a crucial role in neurogenic inflammation and pain transmission, making NK1 receptor antagonists effective in managing conditions such as depression, nausea, and inflammatory diseases. Clinical implications of manipulating these peptides highlight their roles in developing novel analgesics and anti-inflammatory agents with reduced side effects compared to traditional opioids.
Future Research Directions
Future research on dynorphin and tachykinin should prioritize elucidating their distinct receptor subtype interactions and signaling pathways to develop targeted therapeutics for pain and mood disorders. Investigations employing advanced neuroimaging and genetic techniques can clarify their roles in neuroinflammation and neurotransmission modulation. Exploring dynorphin-tachykinin crosstalk at synaptic and systemic levels may uncover novel mechanisms underlying stress responses and neurodegenerative diseases.
Dynorphin Infographic
 libterm.com
libterm.com