Effective filtration removes contaminants from liquids or gases, enhancing purity and performance across various applications. Advanced filtration techniques ensure the removal of particles, bacteria, and chemical impurities, contributing to safety and efficiency in industries like water treatment, pharmaceuticals, and manufacturing. Discover how optimizing your filtration process can improve outcomes by reading the rest of this article.
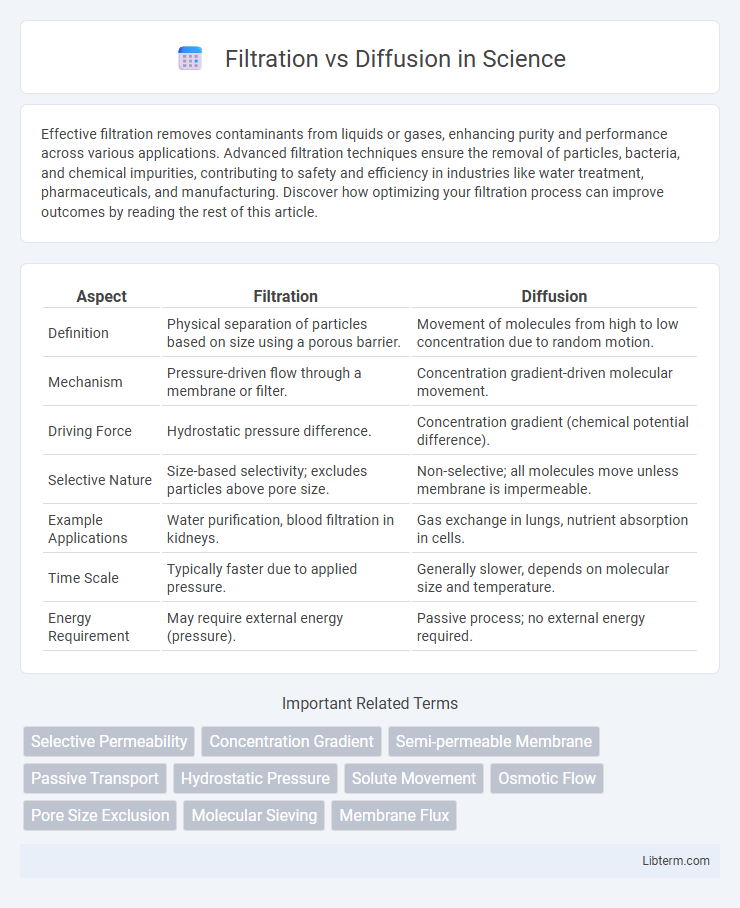
Table of Comparison
| Aspect | Filtration | Diffusion |
|---|---|---|
| Definition | Physical separation of particles based on size using a porous barrier. | Movement of molecules from high to low concentration due to random motion. |
| Mechanism | Pressure-driven flow through a membrane or filter. | Concentration gradient-driven molecular movement. |
| Driving Force | Hydrostatic pressure difference. | Concentration gradient (chemical potential difference). |
| Selective Nature | Size-based selectivity; excludes particles above pore size. | Non-selective; all molecules move unless membrane is impermeable. |
| Example Applications | Water purification, blood filtration in kidneys. | Gas exchange in lungs, nutrient absorption in cells. |
| Time Scale | Typically faster due to applied pressure. | Generally slower, depends on molecular size and temperature. |
| Energy Requirement | May require external energy (pressure). | Passive process; no external energy required. |
Understanding Filtration and Diffusion
Filtration involves the physical removal of particles from a fluid by passing it through a porous medium, relying on pressure differences to separate solids from liquids or gases. Diffusion refers to the movement of molecules from an area of higher concentration to lower concentration driven by concentration gradients without the need for external forces. Understanding filtration is essential in water treatment and air purification, while diffusion plays a critical role in processes like gas exchange in lungs and nutrient transport in cells.
Key Differences Between Filtration and Diffusion
Filtration involves the movement of fluid through a porous membrane driven by pressure, selectively separating particles based on size, while diffusion is the passive movement of molecules from an area of higher concentration to lower concentration without the need for external pressure. Filtration primarily depends on physical barriers such as filters or membranes, whereas diffusion relies on molecular kinetic energy and concentration gradients. Key differences include the driving forces--pressure for filtration and concentration gradient for diffusion--and the types of substances moved, with filtration typically filtering out particles and diffusion facilitating solute movement across membranes.
Mechanisms of Filtration
Filtration operates through a physical barrier that separates particles from fluids based on size exclusion, using mechanisms such as sieving, interception, and inertial impaction to trap contaminants. Pressure differences drive the fluid through porous media like membranes or filter beds, allowing only particles smaller than the pore size to pass. This process contrasts with diffusion, where molecules move from areas of high to low concentration without selective particle retention.
Mechanisms of Diffusion
Diffusion operates through the passive movement of molecules from regions of higher concentration to regions of lower concentration, driven by the concentration gradient without requiring cellular energy. This mechanism relies on random molecular motion and occurs across semipermeable membranes, facilitating the exchange of gases like oxygen and carbon dioxide. Unlike filtration, which depends on hydrostatic pressure to move fluids and solutes, diffusion is governed primarily by kinetic energy and membrane permeability.
Role of Membranes in Filtration and Diffusion
Membranes play a critical role in both filtration and diffusion by selectively controlling the movement of substances based on size, charge, or concentration gradients. In filtration, membranes act as physical barriers that allow the passage of fluids while retaining larger particles or solutes, utilizing pressure differences to drive fluid through pores. In diffusion, membranes permit the passive movement of molecules such as gases or small solutes across them, enabling the equalization of concentration without requiring external energy input.
Real-World Examples of Filtration
Filtration is widely used in water treatment plants to remove contaminants and suspended particles, ensuring safe drinking water. Industrial processes utilize filtration systems to separate solids from liquids, such as in pharmaceutical manufacturing and oil refining. Air filtration in HVAC systems improves indoor air quality by capturing dust, allergens, and pollutants, enhancing health and comfort in residential and commercial buildings.
Real-World Examples of Diffusion
Diffusion plays a critical role in everyday phenomena such as the spread of perfume molecules in air and the exchange of oxygen and carbon dioxide in the lungs. In natural ecosystems, diffusion enables nutrient transport across cell membranes and the movement of gases in aquatic environments. Unlike filtration, which relies on pressure and membranes to separate substances, diffusion depends on concentration gradients, making it essential for passive molecular movement in biological and environmental systems.
Applications in Biology and Medicine
Filtration plays a crucial role in kidney function by enabling selective removal of waste and excess fluids from blood through glomerular membranes. Diffusion drives essential processes like gas exchange in alveoli and nutrient transport across cell membranes, facilitating cellular respiration and metabolism. Both mechanisms underpin drug delivery systems, with filtration aiding in renal clearance rates and diffusion determining drug absorption and distribution at the cellular level.
Advantages and Limitations of Filtration vs Diffusion
Filtration offers precise separation by using a physical barrier to remove particles based on size, making it highly effective for purifying liquids and gases without chemical alteration. However, filtration can be limited by membrane fouling and the inability to separate dissolved substances. Diffusion enables the passive movement of molecules across membranes driven by concentration gradients, allowing selective transport without external energy, but its rate is typically slower and less controlled compared to filtration techniques.
Choosing the Right Process: Filtration or Diffusion
Choosing the right process between filtration and diffusion depends on the specific separation goals and medium properties. Filtration is ideal for separating particles based on size using a porous barrier, effective in liquid-solid or gas-solid separation. Diffusion, governed by concentration gradients and molecular motion, suits applications requiring the passive transport of solutes across membranes without mechanical intervention.
Filtration Infographic
 libterm.com
libterm.com