Catalysis accelerates chemical reactions without being consumed, enabling efficient transformation of reactants with minimal energy input. Stoichiometric reactions, by contrast, consume reactants in exact proportions to produce products, often requiring precise measurement for optimal outcomes. Explore the full article to understand how these concepts impact your approach to chemical processes and experimental design.
Table of Comparison
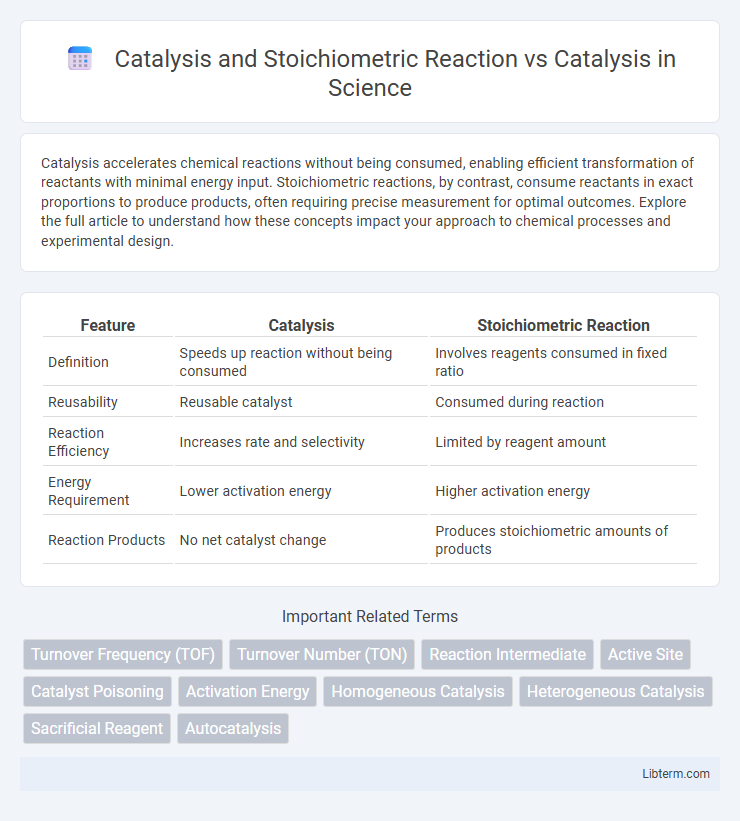
| Feature | Catalysis | Stoichiometric Reaction |
|---|---|---|
| Definition | Speeds up reaction without being consumed | Involves reagents consumed in fixed ratio |
| Reusability | Reusable catalyst | Consumed during reaction |
| Reaction Efficiency | Increases rate and selectivity | Limited by reagent amount |
| Energy Requirement | Lower activation energy | Higher activation energy |
| Reaction Products | No net catalyst change | Produces stoichiometric amounts of products |
Introduction to Catalysis in Chemistry
Catalysis in chemistry involves increasing the rate of a chemical reaction by lowering the activation energy through the presence of a catalyst, which remains unchanged after the reaction. Unlike stoichiometric reactions that consume reactants in fixed proportions to produce products, catalytic processes allow for repeated cycles without depleting the catalyst. Understanding catalysis is fundamental for industrial applications such as the Haber process, where catalysts enable efficient synthesis of ammonia under milder conditions.
Defining Stoichiometric Reactions
Stoichiometric reactions involve reactants combining in exact molar ratios as dictated by a balanced chemical equation, with reactants being completely consumed to form products. Catalysis differs as catalysts participate in the reaction mechanism without being consumed, lowering activation energy and increasing reaction rates without altering stoichiometric coefficients. Understanding stoichiometric reactions is essential for quantifying reactant-product relationships and distinguishing them from catalytic processes where catalysts enable multiple reaction cycles.
Key Principles of Catalysis
Catalysis involves the acceleration of chemical reactions by substances called catalysts that are not consumed in the process, allowing the reaction to reach equilibrium faster without altering overall thermodynamics. Key principles of catalysis include lowering activation energy, providing an alternative reaction pathway, and forming transient intermediates that facilitate product formation. In contrast, stoichiometric reactions rely on reactants reacting in exact molar ratios without catalytic acceleration, resulting in reagent consumption proportional to product formation.
Comparing Catalytic and Stoichiometric Processes
Catalytic processes enable the transformation of reactants into products by lowering the activation energy without being consumed, allowing for multiple reaction cycles from a single catalyst molecule. In contrast, stoichiometric reactions require reactants in precise molar ratios, with each reactant molecule consumed permanently in forming products, resulting in equimolar consumption of reagents and less efficiency. Catalysis optimizes reaction efficiency and sustainability by minimizing waste and enabling continuous turnover, while stoichiometric reactions often produce stoichiometric amounts of byproducts and necessitate reagent replenishment.
Mechanisms of Catalytic Reactions
Catalytic reactions operate through mechanisms that lower activation energy by providing an alternative reaction pathway, involving the formation of transient catalyst-substrate complexes that are regenerated at the end of the cycle. In contrast, stoichiometric reactions consume reactants in fixed proportions without catalyst regeneration, often resulting in complete reactant conversion with no catalytic intermediates. Understanding catalytic mechanisms entails studying steps such as adsorption, surface reaction, and desorption, which facilitate enhanced reaction rates and selectivity compared to stoichiometric processes.
Efficiency and Selectivity in Catalysis vs Stoichiometry
Catalysis enhances reaction efficiency by lowering activation energy and increasing reaction rates without being consumed, unlike stoichiometric reactions that rely on one-to-one reagent consumption, resulting in higher resource use and waste. Catalytic processes offer superior selectivity by enabling specific reaction pathways and minimizing side products, whereas stoichiometric reactions often produce more byproducts due to less controlled mechanisms. This difference makes catalysis more sustainable, cost-effective, and preferable in industrial applications requiring precision and material conservation.
Environmental Impact: Catalysis Versus Stoichiometric Reactions
Catalysis significantly reduces environmental impact by enabling chemical reactions to proceed with lower energy consumption and minimal waste generation compared to stoichiometric reactions, which often require excess reagents that produce large volumes of hazardous byproducts. Catalysts facilitate multiple reaction cycles without being consumed, improving atom economy and reducing the overall volume of chemical waste. This efficiency decreases resource depletion and lowers the ecological footprint of industrial processes, making catalysis a critical strategy in sustainable chemistry.
Industrial Applications of Catalysis
Catalysis accelerates chemical reactions by lowering activation energy without being consumed, enabling efficient industrial processes like ammonia synthesis via the Haber-Bosch method and petroleum refining through catalytic cracking. In contrast, stoichiometric reactions consume reactants in fixed proportions, often producing more waste and requiring higher energy inputs, limiting their industrial scalability. Industrial applications favor catalysis for enhanced selectivity, reduced operating costs, and improved sustainability in large-scale chemical manufacturing.
Challenges and Limitations in Both Processes
Catalysis often faces challenges such as catalyst deactivation, limited selectivity, and the need for precise reaction conditions to achieve high efficiency. Stoichiometric reactions, in contrast, are limited by reagent consumption and waste generation, making them less sustainable for large-scale applications. Both processes struggle with scalability and maintaining reaction control, but catalysis offers better potential for reuse and lower environmental impact despite its complexity.
Future Trends in Catalysis and Stoichiometric Chemistry
Future trends in catalysis emphasize the development of sustainable, efficient catalysts using earth-abundant metals and bio-inspired design to enhance reaction specificity and reduce environmental impact. Advances in computational chemistry and machine learning are accelerating catalyst discovery, optimizing reaction conditions, and enabling precise control over catalytic cycles in stoichiometric chemistry. Integration of electrochemical and photochemical methods with traditional catalytic processes is driving innovation toward greener, energy-efficient chemical synthesis and industrial applications.
Catalysis and Stoichiometric Reaction Infographic
 libterm.com
libterm.com