Super-saturated solutions contain more dissolved solute than typically possible under standard conditions, creating a state of instability that can lead to rapid crystallization. Understanding how super-saturation influences chemical reactions and material properties is crucial for applications in pharmaceuticals, food science, and manufacturing. Explore the full article to discover how super-saturation impacts your processes and innovations.
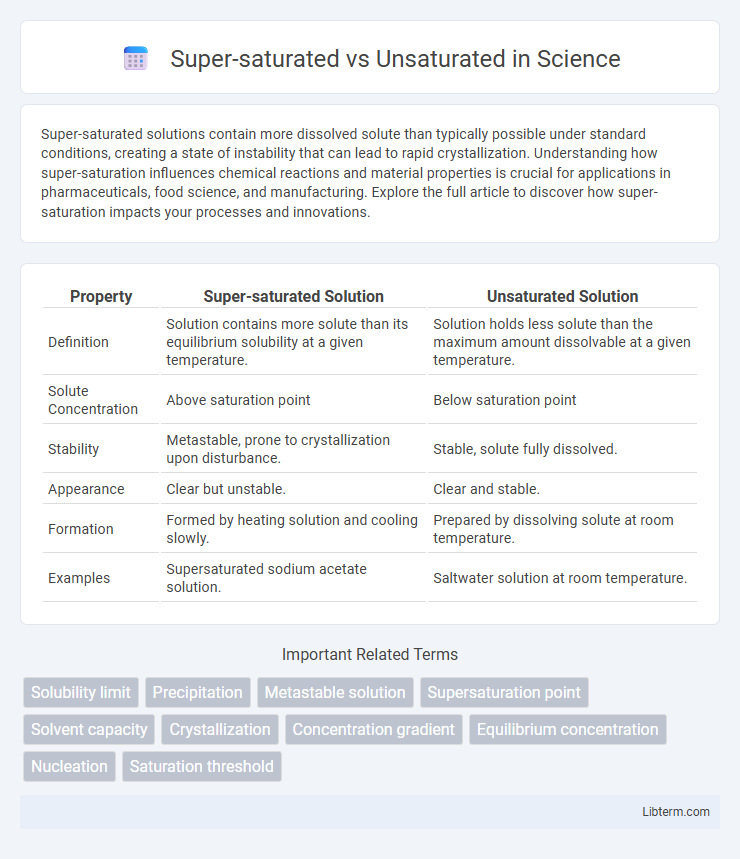
Table of Comparison
| Property | Super-saturated Solution | Unsaturated Solution |
|---|---|---|
| Definition | Solution contains more solute than its equilibrium solubility at a given temperature. | Solution holds less solute than the maximum amount dissolvable at a given temperature. |
| Solute Concentration | Above saturation point | Below saturation point |
| Stability | Metastable, prone to crystallization upon disturbance. | Stable, solute fully dissolved. |
| Appearance | Clear but unstable. | Clear and stable. |
| Formation | Formed by heating solution and cooling slowly. | Prepared by dissolving solute at room temperature. |
| Examples | Supersaturated sodium acetate solution. | Saltwater solution at room temperature. |
Introduction to Saturation in Chemistry
Saturation in chemistry refers to the extent to which a solution contains the maximum amount of dissolved solute at a given temperature and pressure. A super-saturated solution contains more dissolved solute than its equilibrium solubility, making it unstable and prone to precipitation. An unsaturated solution has less dissolved solute than its saturation point, allowing more solute to dissolve without reaching equilibrium.
Defining Super-Saturated and Unsaturated Solutions
Super-saturated solutions contain more dissolved solute than the equilibrium concentration at a given temperature, resulting in a metastable state that can rapidly crystallize upon disturbance. Unsaturated solutions have less solute dissolved than the maximum capacity, allowing more solute to dissolve until saturation is reached. The concentration level relative to the solubility limit distinguishes these two solution types.
Key Differences: Super-Saturated vs Unsaturated
Super-saturated solutions contain more solute than the maximum amount that can typically dissolve at a given temperature, making them unstable and prone to crystallization. Unsaturated solutions have less solute than the solvent's capacity, allowing for further dissolution without precipitation. The key difference lies in solute concentration relative to the solvent's equilibrium capacity, impacting stability and crystallization behavior.
Formation of Super-Saturated Solutions
Super-saturated solutions form when a solvent holds more solute than its normal saturation point at a given temperature, achieved by dissolving solute at elevated temperatures followed by slow cooling. This metastable state requires careful handling to prevent rapid crystallization, as even minor disturbances can trigger solute precipitation. Unlike unsaturated solutions, super-saturated solutions exceed equilibrium solubility, creating unique conditions for crystal growth and nucleation studies.
Properties of Unsaturated Solutions
Unsaturated solutions contain less solute than the maximum amount that can dissolve at a given temperature, resulting in a stable, clear mixture without precipitation. These solutions exhibit dynamic equilibrium where solute particles continuously dissolve and crystallize, maintaining solubility limits. Physical properties such as boiling point, freezing point, and vapor pressure remain similar to pure solvent but are slightly altered based on solute concentration within the unsaturation range.
Factors Affecting Saturation Levels
Solubility depends on temperature, pressure, and the nature of solutes and solvents, significantly influencing saturation levels. Higher temperatures generally increase solubility for solids, resulting in super-saturated solutions when exceeded saturation points cool slowly. Pressure mainly affects gases, with increased pressure raising gas solubility and thus impacting solutions' saturation state.
Examples of Super-Saturated Solutions
Super-saturated solutions contain more dissolved solute than the solvent's normal capacity at a given temperature, creating unstable and highly concentrated mixtures. Examples of super-saturated solutions include sodium acetate in hot water, where cooling results in a clear, metastable solution used in hand warmers, and potassium nitrate dissolved in hot water that crystallizes upon cooling, often utilized in crystallization demonstration experiments. These solutions contrast with unsaturated solutions, which hold less solute than the maximum capacity, such as sugar dissolved incrementally in water at room temperature.
Real-World Applications of Unsaturated Solutions
Unsaturated solutions are widely used in pharmaceuticals where precise dosing and solubility control are critical for drug formulation and bioavailability. In environmental science, unsaturated soils influence water retention and nutrient availability, impacting agriculture and ecosystem health. Industrial processes such as textile dyeing utilize unsaturated dye solutions to achieve consistent color absorption and material quality.
Common Experiments: Observing Saturation States
Common experiments observing saturation states involve dissolving solids in solvents at varying temperatures to determine the solubility limit, creating saturated solutions where no more solute dissolves. When additional solute is added beyond this point without dissolving, a super-saturated solution forms, often prepared by heating a saturated solution and then slowly cooling it. These experiments typically include crystallization tests to visually confirm super-saturation and demonstrate the instability of such solutions compared to stable unsaturated or saturated states.
Conclusion: Choosing Between Super-Saturated and Unsaturated
Choosing between super-saturated and unsaturated solutions depends on the desired chemical stability and application requirements. Super-saturated solutions offer higher solute concentrations but are thermodynamically unstable and prone to crystallization, making them suitable for controlled precipitation processes. Unsaturated solutions provide greater stability and ease of handling, ideal for routine chemical reactions and formulations requiring consistent solubility.
Super-saturated Infographic
 libterm.com
libterm.com