Kinetic energy describes the energy an object possesses due to its motion, playing a crucial role in physics and engineering. Understanding kinetic principles helps optimize mechanical systems, improve safety, and enhance performance across various applications. Dive into the rest of this article to explore how kinetic energy impacts your daily life and technological advancements.
Table of Comparison
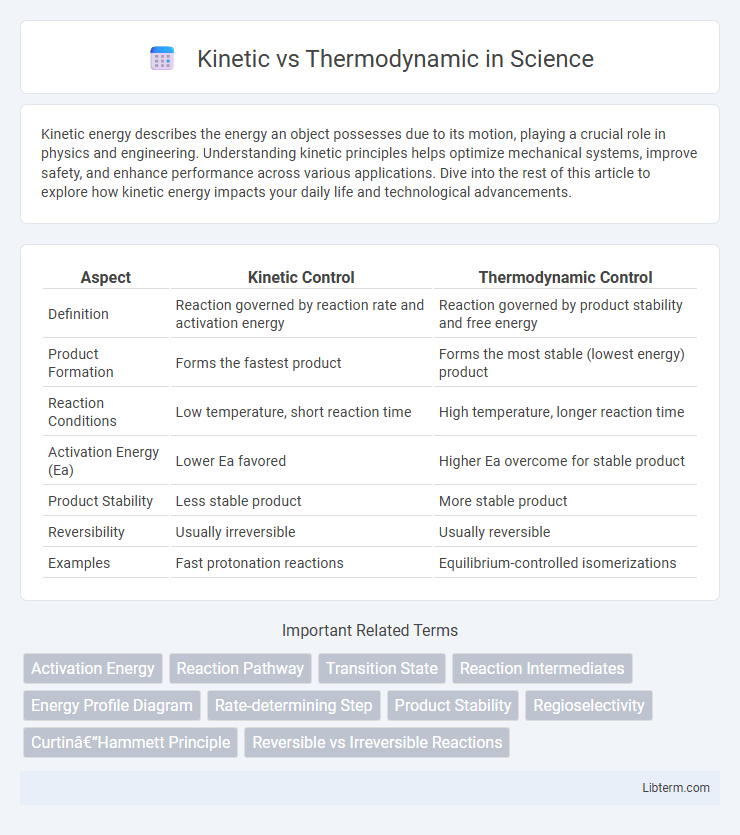
| Aspect | Kinetic Control | Thermodynamic Control |
|---|---|---|
| Definition | Reaction governed by reaction rate and activation energy | Reaction governed by product stability and free energy |
| Product Formation | Forms the fastest product | Forms the most stable (lowest energy) product |
| Reaction Conditions | Low temperature, short reaction time | High temperature, longer reaction time |
| Activation Energy (Ea) | Lower Ea favored | Higher Ea overcome for stable product |
| Product Stability | Less stable product | More stable product |
| Reversibility | Usually irreversible | Usually reversible |
| Examples | Fast protonation reactions | Equilibrium-controlled isomerizations |
Introduction to Kinetic and Thermodynamic Control
Kinetic control governs product formation based on the reaction rate, favoring products that form faster but may be less stable. Thermodynamic control favors the most stable, lowest energy product formed under equilibrium conditions, regardless of formation speed. Understanding the balance between kinetic and thermodynamic control is critical for manipulating reaction outcomes in synthetic chemistry.
Defining Kinetic Products vs Thermodynamic Products
Kinetic products form faster due to lower activation energy and are favored under conditions of lower temperature or shorter reaction times, often resulting in less stable compounds. Thermodynamic products arise from reactions that reach equilibrium, producing the most stable compounds with lower overall free energy despite requiring higher activation energy and longer reaction times. Understanding the distinction between kinetic and thermodynamic products is crucial for controlling reaction pathways in synthetic chemistry and optimizing yield selectivity.
Reaction Pathways: How Conditions Influence Outcomes
Kinetic control favors the fastest reaction pathway, producing products that form quickly but may be less stable, commonly observed at lower temperatures or short reaction times. Thermodynamic control leads to the most stable product, favored under higher temperatures or longer reaction times where equilibrium is reached. Reaction conditions such as temperature, pressure, and catalysts determine whether kinetic or thermodynamic pathways dominate, directly impacting product distribution and yield.
Activation Energy and Reaction Rates
Kinetic control in chemical reactions is determined by lower activation energy, leading to faster reaction rates and the formation of products favored under rapid conditions. Thermodynamic control depends on the overall stability of products, favoring those with lower free energy but typically involving higher activation energy and slower reaction rates. Understanding the balance between activation energy and reaction rates is crucial for predicting product distribution in kinetically versus thermodynamically controlled processes.
Stability and Energy Profiles of Products
Kinetic products form faster due to lower activation energy but are less stable and higher in energy compared to thermodynamic products, which require higher activation energy and form more slowly but result in greater stability and lower energy. Energy profiles show kinetic pathways having smaller energy barriers leading to less stable, higher-energy intermediates, whereas thermodynamic pathways traverse higher activation barriers but reach the most stable, low-energy products. Stability differences arise from product energy minimization, with thermodynamic control favoring equilibrium-favored compounds and kinetic control favoring products under rapid or low-temperature conditions.
Factors Affecting Kinetic and Thermodynamic Selectivity
Kinetic selectivity is influenced primarily by reaction conditions such as temperature, catalyst presence, and activation energy barriers, favoring products formed fastest under lower energy pathways. Thermodynamic selectivity depends on the relative stability of final products, driven by enthalpy and entropy differences, where equilibrium conditions favor the most stable product with the lowest Gibbs free energy (DG). Factors like reaction time and reversibility determine whether kinetic or thermodynamic control dominates, affecting product distribution in chemical reactions.
Examples in Organic Chemistry Synthesis
In organic chemistry synthesis, kinetic products form faster due to lower activation energy but are often less stable, such as the 1,2-addition of HBr to conjugated dienes producing allylic bromides at low temperatures. Thermodynamic products, like the 1,4-addition in the same reaction, form more slowly but are more stable, favored at higher temperatures due to greater reaction reversibility. Another example includes enolate formation where kinetic enolates form quickly under sterically hindered, strong bases at low temperatures, while thermodynamic enolates predominate under equilibrating conditions and higher temperatures, offering the most substituted alkene.
Key Experimental Conditions: Temperature and Time
Kinetic control favors product formation at lower temperatures and shorter reaction times, where activation energy barriers dictate product distribution. Thermodynamic control is achieved at higher temperatures and longer reaction times, allowing the system to reach equilibrium and form the most stable product. Precise temperature regulation and reaction duration are crucial to selectively obtaining kinetic or thermodynamic products in chemical synthesis.
Practical Implications in Industrial and Pharmaceutical Processes
Kinetic control in industrial and pharmaceutical processes favors the formation of products that form fastest, often at lower temperatures, enabling rapid synthesis of unstable intermediates crucial for drug development. Thermodynamic control drives the formation of the most stable products, achieved by allowing reactions to reach equilibrium under higher temperatures or longer reaction times, essential for producing durable materials and optimizing yield. Understanding the balance between kinetic and thermodynamic factors is critical for maximizing efficiency, selectivity, and cost-effectiveness in large-scale chemical manufacturing and pharmaceutical formulation.
Summary and Key Takeaways
Kinetic control favors the product with the lowest activation energy, forming faster but possibly less stable compounds, while thermodynamic control yields the most stable product, favored at equilibrium due to lower overall free energy. Kinetic products dominate at lower temperatures and shorter reaction times, whereas thermodynamic products are favored at higher temperatures and longer reaction durations. Understanding the balance between kinetic and thermodynamic control is essential for predicting reaction outcomes and manipulating product distributions in chemical synthesis.
Kinetic Infographic
 libterm.com
libterm.com