Neutralization is a chemical reaction where an acid and a base interact to form water and a salt, effectively canceling each other's properties. This process plays a crucial role in various applications, from industrial waste treatment to biological systems maintaining pH balance. Explore the rest of the article to understand how neutralization impacts everyday life and your environment.
Table of Comparison
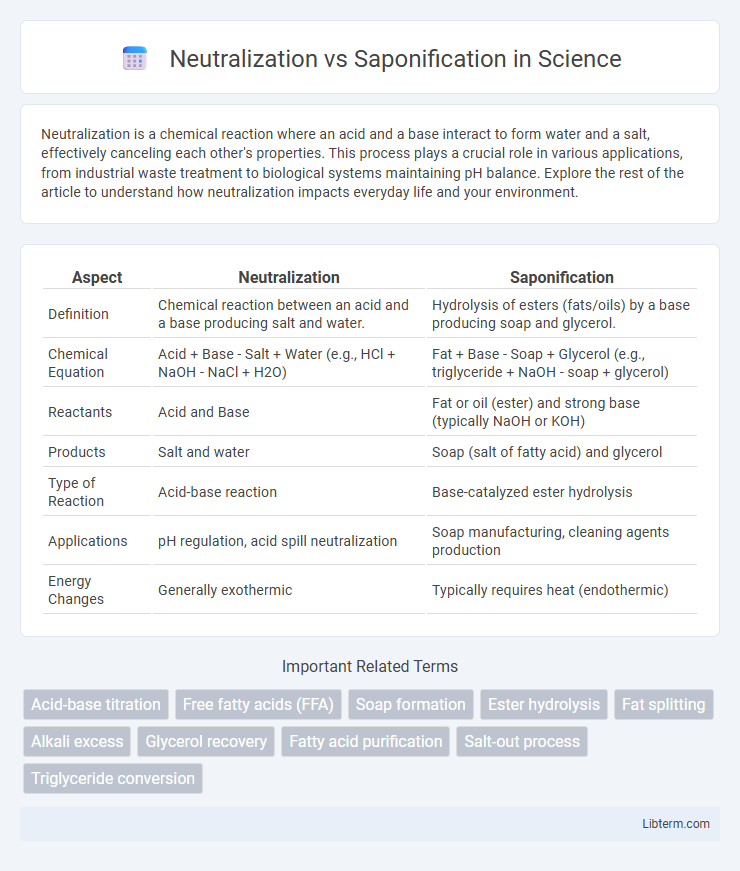
| Aspect | Neutralization | Saponification |
|---|---|---|
| Definition | Chemical reaction between an acid and a base producing salt and water. | Hydrolysis of esters (fats/oils) by a base producing soap and glycerol. |
| Chemical Equation | Acid + Base - Salt + Water (e.g., HCl + NaOH - NaCl + H2O) | Fat + Base - Soap + Glycerol (e.g., triglyceride + NaOH - soap + glycerol) |
| Reactants | Acid and Base | Fat or oil (ester) and strong base (typically NaOH or KOH) |
| Products | Salt and water | Soap (salt of fatty acid) and glycerol |
| Type of Reaction | Acid-base reaction | Base-catalyzed ester hydrolysis |
| Applications | pH regulation, acid spill neutralization | Soap manufacturing, cleaning agents production |
| Energy Changes | Generally exothermic | Typically requires heat (endothermic) |
Understanding Neutralization: Basic Concepts
Neutralization involves the chemical reaction between an acid and a base, producing water and a salt, which is fundamental in controlling pH levels in various industrial and biological processes. This reaction is typically exothermic and reaches completion when the amount of acid equals the amount of base, known as the equivalence point. Understanding neutralization is crucial in fields such as pharmaceuticals, environmental science, and food chemistry, where maintaining appropriate pH levels ensures product stability and safety.
Saponification Explained: Definition and Process
Saponification is a chemical reaction that occurs when a fat or oil reacts with a strong base, such as sodium hydroxide or potassium hydroxide, resulting in the formation of soap and glycerol. This process involves the hydrolysis of triglycerides into fatty acid salts, which act as soap molecules with cleansing properties. Compared to neutralization, saponification specifically targets esters in fats and oils, producing a valuable product used in detergents, cosmetics, and industrial applications.
Chemical Reactions: Comparing Mechanisms
Neutralization involves the reaction between an acid and a base to produce water and a salt, typically following the mechanism of proton transfer from the acid to the base. Saponification is a specific type of base-catalyzed ester hydrolysis where triglycerides react with a strong base, such as sodium hydroxide, yielding glycerol and soap (fatty acid salts). While both processes involve base interactions, neutralization is a straightforward acid-base reaction, whereas saponification entails breaking ester bonds through nucleophilic attack by hydroxide ions.
Key Components: Acids, Bases, and Fats
Neutralization involves the reaction between acids and bases to form water and salt, focusing on the complete neutralization of hydrogen ions by hydroxide ions. Saponification specifically requires a strong base, such as sodium hydroxide, reacting with fats or triglycerides to produce glycerol and soap molecules. Key components in saponification include fatty acids derived from fats that are hydrolyzed, distinguishing it from general neutralization which does not involve lipid substrates.
Industrial Applications of Neutralization
Neutralization reactions are widely applied in industrial processes to control pH levels in wastewater treatment, chemical manufacturing, and metal refining, ensuring safe discharge and product stability. Unlike saponification, which specifically produces soap through the reaction of fats with alkali, neutralization broadly involves acid-base reactions that neutralize harmful substances and optimize reaction conditions. Industrial neutralization enhances environmental compliance and operational efficiency by converting hazardous acidic or alkaline waste streams into neutral compounds.
Uses of Saponification in Everyday Life
Saponification is widely used in everyday life to produce soap, where fats or oils react with a strong alkali like sodium hydroxide to create glycerol and soap, essential for personal hygiene and cleaning. This process also enables the manufacturing of detergents and various cosmetics, enhancing skin care and cleanliness. Unlike neutralization, which primarily balances pH in chemical reactions, saponification directly transforms fats into valuable cleaning agents critical for domestic and industrial applications.
Differences in End Products
Neutralization produces water and a salt through the reaction of an acid and a base, resulting in a neutral or near-neutral solution. Saponification specifically involves the reaction of a fat or oil with a strong base, typically sodium hydroxide, yielding glycerol and soap as end products. The key difference in end products is that neutralization forms simple salts and water, while saponification generates soap molecules and glycerol, which are distinct compounds with differing applications.
Environmental Impact: Neutralization vs Saponification
Neutralization typically produces salt and water with minimal hazardous byproducts, making it more environmentally benign compared to saponification, which generates soap and glycerol that require careful waste management. Saponification processes often involve the use of strong alkalis like sodium hydroxide, resulting in higher chemical energy consumption and potential ecological toxicity if not properly treated. Neutralization's simpler chemical reactions generally lead to lower carbon footprints and reduced soil and water contamination risks, favoring its application in eco-conscious industries.
Safety Precautions and Handling
Neutralization involves the controlled addition of acids or bases, requiring appropriate personal protective equipment (PPE) such as gloves and goggles to prevent chemical burns and respiratory irritation. Saponification, the process of reacting fats with lye (sodium hydroxide), demands careful handling of caustic substances to avoid skin contact and inhalation hazards. Proper ventilation, use of chemical-resistant containers, and immediate access to safety showers are critical when managing these chemical reactions to minimize risk.
Summary Table: Neutralization vs Saponification
Neutralization involves the reaction between an acid and a base to form water and a salt, while saponification specifically refers to the base-catalyzed hydrolysis of esters, such as fats or oils, producing glycerol and soap. The summary table highlights that neutralization is a broad acid-base reaction applicable to various acid and base pairs, whereas saponification is a specialized neutralization targeting triglycerides with strong bases like sodium hydroxide. Key differences include reaction conditions, products formed, and industrial applications, with neutralization used in pH control and saponification essential in soap manufacturing.
Neutralization Infographic
 libterm.com
libterm.com