Substrate serves as the foundational layer or material upon which processes, substances, or structures are built, playing a crucial role in fields ranging from biology to electronics. Its properties directly impact the performance and durability of the systems dependent on it, making selection and understanding essential for optimal results. Explore the article to uncover the various applications and significance of substrate in diverse industries.
Table of Comparison
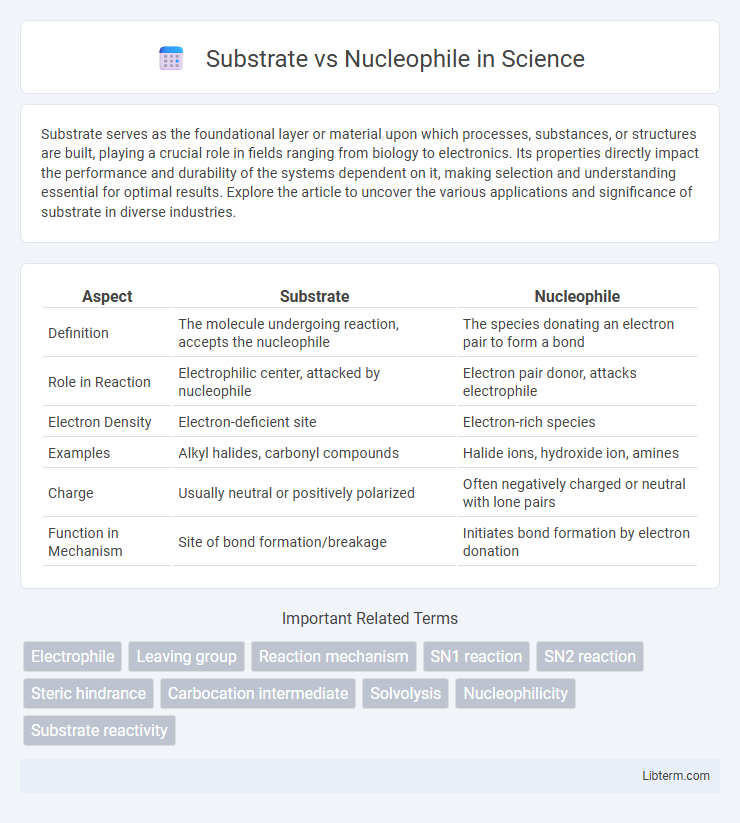
| Aspect | Substrate | Nucleophile |
|---|---|---|
| Definition | The molecule undergoing reaction, accepts the nucleophile | The species donating an electron pair to form a bond |
| Role in Reaction | Electrophilic center, attacked by nucleophile | Electron pair donor, attacks electrophile |
| Electron Density | Electron-deficient site | Electron-rich species |
| Examples | Alkyl halides, carbonyl compounds | Halide ions, hydroxide ion, amines |
| Charge | Usually neutral or positively polarized | Often negatively charged or neutral with lone pairs |
| Function in Mechanism | Site of bond formation/breakage | Initiates bond formation by electron donation |
Introduction to Substrate and Nucleophile
A substrate is the molecule undergoing a chemical reaction, typically consisting of specific functional groups that interact with reagents or catalysts. A nucleophile is an electron-rich species that donates a pair of electrons to form a new covalent bond with an electrophilic center on the substrate. Understanding the nature of both substrate and nucleophile is essential for predicting reaction mechanisms and outcomes in organic chemistry.
Defining Substrate in Organic Reactions
A substrate in organic reactions is the specific molecule undergoing transformation when interacting with reagents, often characterized by distinct reactive sites such as electrophilic carbons or double bonds. It serves as the foundation upon which nucleophiles act, donating electron pairs to form new chemical bonds during reaction mechanisms. Understanding substrate structure is crucial for predicting reaction pathways and the outcome of nucleophilic attacks.
What is a Nucleophile?
A nucleophile is a chemical species that donates an electron pair to form a covalent bond during a chemical reaction. It is electron-rich and seeks positively charged or electron-deficient centers, commonly attacking electrophilic substrates. Common nucleophiles include hydroxide ions, amines, and halide ions, impacting reaction mechanisms such as nucleophilic substitution.
Structural Differences: Substrate vs Nucleophile
Substrates typically contain electrophilic centers such as carbon atoms attached to leaving groups, making them susceptible to nucleophilic attack, while nucleophiles possess lone pairs or negative charges enabling them to donate electrons. The structural difference lies in substrates often having sp3, sp2, or sp hybridized carbons bonded to leaving groups, whereas nucleophiles include atoms like oxygen, nitrogen, sulfur, or carbon with electron-rich sites. This distinction in electron density and bonding arrangement governs the mechanistic pathway of substitution and elimination reactions.
Role of Substrates in Chemical Reactions
Substrates serve as the primary reactants in chemical reactions, providing the molecular framework to which nucleophiles attach or replace groups during substitution processes. The nature of the substrate--whether primary, secondary, or tertiary--significantly influences the reaction mechanism, favoring either SN1 or SN2 pathways based on steric hindrance and carbocation stability. Substrate structure dictates reaction rate and product outcome by affecting nucleophile accessibility and intermediate formation in nucleophilic substitution reactions.
Nucleophile Function and Reactivity
Nucleophiles act as electron-rich species that donate a pair of electrons to electrophilic centers on substrates, initiating chemical reactions such as substitution or addition. Their reactivity is influenced by factors including charge, electronegativity, solvent effects, and steric hindrance, which determine their ability to attack the substrate's reactive sites. Comparing nucleophiles involves assessing these properties to predict reaction rates and mechanisms in organic synthesis.
Factors Affecting Substrate Reactivity
Substrate reactivity in nucleophilic substitution reactions is influenced by the nature of the leaving group, the steric hindrance around the reactive site, and the hybridization of the carbon atom involved. Primary substrates typically react faster in SN2 mechanisms due to minimal steric hindrance, whereas tertiary substrates favor SN1 reactions because of carbocation stability. The electronegativity of substituents adjacent to the reactive center can also stabilize or destabilize the transition state, significantly affecting the overall reaction rate.
Factors Influencing Nucleophile Strength
Nucleophile strength is influenced by factors such as charge, electronegativity, solvent effects, and steric hindrance, each impacting its ability to donate electron pairs to the substrate. Negatively charged nucleophiles typically exhibit greater strength than their neutral counterparts due to increased electron density. Polar protic solvents stabilize nucleophiles through hydrogen bonding, reducing nucleophilicity, whereas polar aprotic solvents enhance nucleophile reactivity by lessening solvation effects.
Common Examples of Substrates and Nucleophiles
Common substrates in nucleophilic substitution reactions include alkyl halides, such as methyl bromide and ethyl chloride, as well as tosylates and alkyl sulfonates. Typical nucleophiles consist of negatively charged species like hydroxide (OH-), cyanide (CN-), and alkoxides (RO-), along with neutral molecules such as ammonia (NH3) and water (H2O). Understanding the interaction between substrates and nucleophiles is crucial for predicting reaction pathways in organic synthesis.
Substrate-Nucleophile Interactions in Reaction Mechanisms
Substrate-nucleophile interactions play a crucial role in determining the course and rate of organic reaction mechanisms, especially in nucleophilic substitution reactions like SN1 and SN2. The electronic and steric properties of the substrate influence nucleophile access and attack, with primary substrates favoring backside attack in SN2 processes, whereas tertiary substrates typically undergo carbocation formation leading to SN1 pathways. Understanding the interplay between substrate structure and nucleophile strength helps predict reaction outcomes and optimize conditions for efficient chemical synthesis.
Substrate Infographic
 libterm.com
libterm.com