Electrolytes are substances that dissociate into ions in solution, enabling the conduction of electric current, while acids are a specific type of electrolyte that donate protons (H+ ions) in aqueous solutions. Understanding the differences between electrolytes and acids is crucial for grasping key concepts in chemistry, including pH balance and electrical conductivity. Explore the full article to deepen your knowledge about how these substances interact and influence various chemical processes.
Table of Comparison
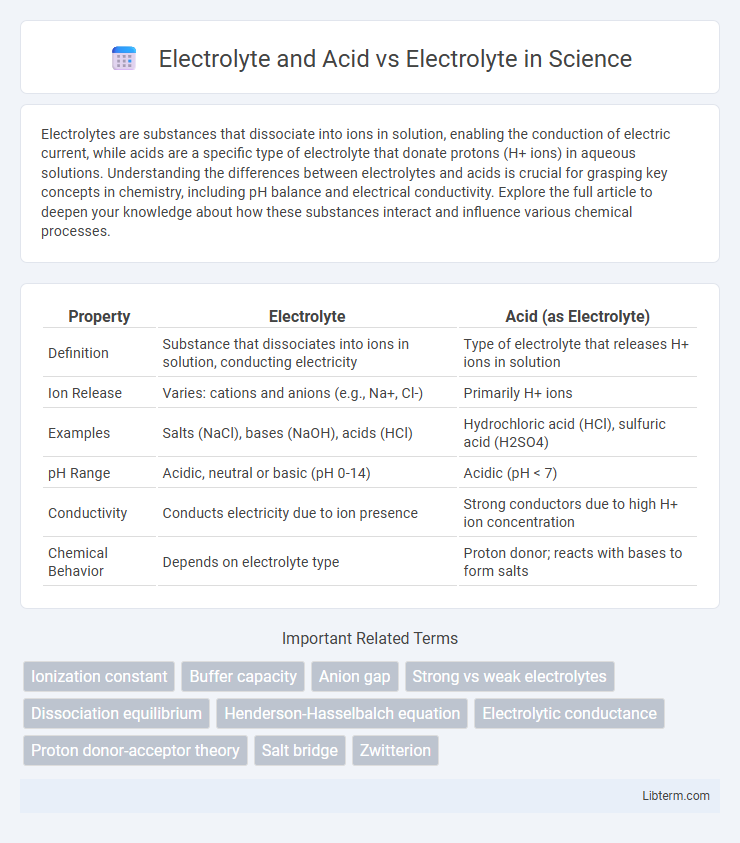
| Property | Electrolyte | Acid (as Electrolyte) |
|---|---|---|
| Definition | Substance that dissociates into ions in solution, conducting electricity | Type of electrolyte that releases H+ ions in solution |
| Ion Release | Varies: cations and anions (e.g., Na+, Cl-) | Primarily H+ ions |
| Examples | Salts (NaCl), bases (NaOH), acids (HCl) | Hydrochloric acid (HCl), sulfuric acid (H2SO4) |
| pH Range | Acidic, neutral or basic (pH 0-14) | Acidic (pH < 7) |
| Conductivity | Conducts electricity due to ion presence | Strong conductors due to high H+ ion concentration |
| Chemical Behavior | Depends on electrolyte type | Proton donor; reacts with bases to form salts |
Understanding Electrolytes: Definition and Functions
Electrolytes are minerals that dissolve in water to produce ions, enabling the conduction of electrical signals essential for bodily functions such as nerve impulses and muscle contractions. Acids, as electrolytes, dissociate in solutions to release hydrogen ions (H+), influencing the solution's pH and conductivity, while general electrolytes include both acids and bases that maintain fluid balance and cellular activity. Understanding the roles of electrolytes involves recognizing their contribution to maintaining homeostasis, regulating hydration, and supporting biochemical reactions in the body.
Types of Electrolytes: Strong vs Weak
Strong electrolytes completely dissociate into ions in aqueous solutions, resulting in high electrical conductivity, examples include hydrochloric acid (HCl) and sodium chloride (NaCl). Weak electrolytes partially dissociate, producing fewer ions and lower conductivity, with acetic acid (CH3COOH) serving as a common example. Understanding the differences in ionization between strong and weak electrolytes is crucial for applications in chemical reactions, batteries, and biological systems.
Acidic Electrolytes: Characteristics and Examples
Acidic electrolytes are substances that release hydrogen ions (H+) in solution, resulting in a low pH and high electrical conductivity. Common examples include sulfuric acid (H2SO4) and hydrochloric acid (HCl), which are widely used in batteries and industrial processes. Their strong ionization properties distinguish acidic electrolytes from neutral or basic electrolytes, influencing their reactivity and applications.
Electrolytes in Acid-Base Balance
Electrolytes such as sodium, potassium, chloride, and bicarbonate play a crucial role in maintaining acid-base balance by regulating the pH of bodily fluids. In acidic conditions, bicarbonate ions neutralize excess hydrogen ions, while electrolyte transport across cell membranes adjusts to restore equilibrium. Proper electrolyte levels are vital for enzymatic functions and nerve impulses, ensuring stable metabolic processes and homeostasis.
Acid vs Electrolyte: Key Differences
Acid and electrolyte differ fundamentally in their chemical properties and functions; acids are substances that release hydrogen ions (H+) in solution, resulting in a low pH, while electrolytes are minerals that dissociate into ions, conducting electricity in body fluids. Electrolytes include a variety of ions such as sodium, potassium, calcium, and chloride, essential for nerve function, muscle contraction, and maintaining fluid balance. Unlike general electrolytes, acids specifically increase hydrogen ion concentration, influencing acidity but may also act as electrolytes if they ionize in solution.
Role of Electrolytes in Biological Systems
Electrolytes, including acids, bases, and salts, dissociate into ions that conduct electrical signals essential for cellular functions in biological systems. They regulate nerve impulses, muscle contractions, hydration, and pH balance, maintaining homeostasis and supporting metabolic processes. Acidic electrolytes contribute hydrogen ions that influence enzyme activity and acid-base equilibrium critical to physiological stability.
How Acids Function as Electrolytes
Acids function as electrolytes by dissociating into ions when dissolved in water, which allows the solution to conduct electricity. The hydrogen ions (H+) released contribute to the increased conductivity, distinguishing acids from other electrolytes like salts and bases. This ionization process is essential for acid strength and their role in chemical reactions and electrochemical applications.
Electrolyte Solutions: Properties and Conductivity
Electrolyte solutions consist of ions that dissociate in water, increasing electrical conductivity by allowing charge to flow freely. Acidic electrolytes specifically contain excess hydrogen ions (H+), which enhance conductivity compared to neutral electrolyte solutions due to higher proton mobility. The concentration, type of ions, and degree of dissociation directly influence the conductivity and other properties such as pH and ionic strength of electrolyte solutions.
Common Misconceptions: Acid vs Electrolyte
Electrolytes are substances that dissociate into ions in solution, enabling electrical conductivity, while acids specifically increase hydrogen ion (H+) concentration in water. A common misconception is that all electrolytes are acids, but many electrolytes, like sodium chloride or potassium hydroxide, are salts or bases rather than acids. Understanding the distinction is crucial, as acids influence pH and chemical reactivity uniquely compared to other electrolytes.
Practical Applications: Electrolytes and Acids in Everyday Life
Electrolytes such as sodium, potassium, and calcium play a crucial role in maintaining hydration, nerve function, and muscle contractions in the human body. Acids like citric acid and acetic acid are commonly used in food preservation, cleaning products, and industrial processes due to their ability to donate protons and alter pH levels. Practical applications of electrolytes encompass sports drinks and medical IV fluids, while acids are essential in manufacturing, agriculture, and household cleaning.
Electrolyte and Acid Infographic
 libterm.com
libterm.com