Condensation occurs when water vapor in the air cools and changes into liquid droplets, often forming on surfaces like windows and walls. This process is a common cause of dampness and mold problems inside buildings, affecting indoor air quality and structural integrity. Discover effective methods to prevent condensation and protect your home by exploring the rest of this article.
Table of Comparison
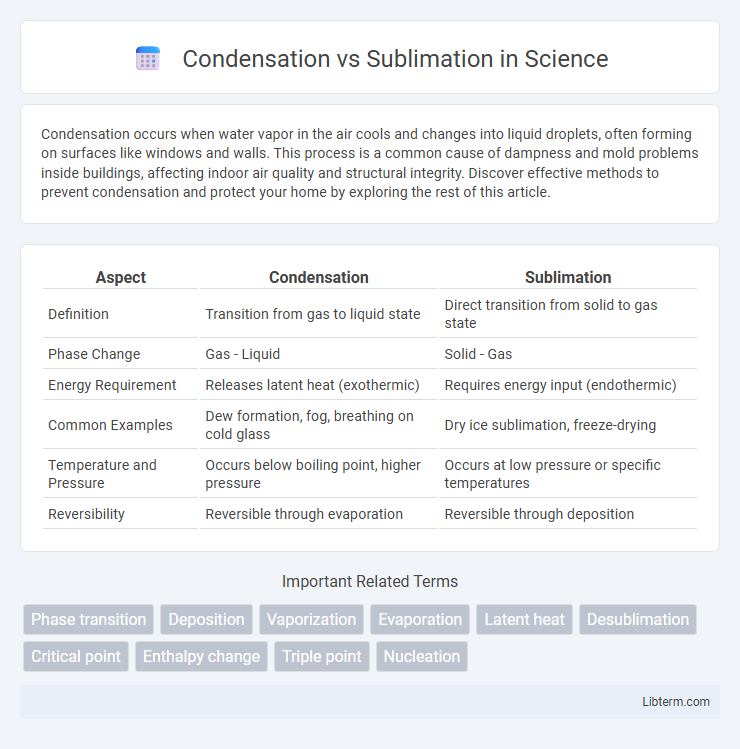
| Aspect | Condensation | Sublimation |
|---|---|---|
| Definition | Transition from gas to liquid state | Direct transition from solid to gas state |
| Phase Change | Gas - Liquid | Solid - Gas |
| Energy Requirement | Releases latent heat (exothermic) | Requires energy input (endothermic) |
| Common Examples | Dew formation, fog, breathing on cold glass | Dry ice sublimation, freeze-drying |
| Temperature and Pressure | Occurs below boiling point, higher pressure | Occurs at low pressure or specific temperatures |
| Reversibility | Reversible through evaporation | Reversible through deposition |
Introduction to Phase Changes
Condensation is the phase change where a gas transforms into a liquid, releasing latent heat as molecules lose energy and come closer together. Sublimation occurs when a solid directly changes into a gas without passing through the liquid state, requiring significant energy input to overcome molecular bonds. These phase changes illustrate the energy dynamics and molecular behavior fundamental to thermodynamics and material science.
What is Condensation?
Condensation is the process where water vapor in the air changes into liquid water when it cools down, commonly seen as dew or fog. This phase transition releases latent heat into the surrounding environment, playing a crucial role in weather patterns and the water cycle. Condensation occurs when air becomes saturated with moisture, typically at the dew point temperature, leading to cloud formation and precipitation.
What is Sublimation?
Sublimation is the phase transition where a substance changes directly from a solid to a gas without passing through the liquid state, occurring under specific temperature and pressure conditions. This process is commonly observed in dry ice (solid carbon dioxide), which sublimates at -78.5degC under atmospheric pressure. Sublimation plays a critical role in various industrial applications such as freeze-drying and in the natural water cycle through the direct evaporation of snow or ice.
Key Differences Between Condensation and Sublimation
Condensation is the phase change where a gas transforms into a liquid, typically occurring when vapor cools below its dew point, whereas sublimation involves a solid changing directly into a gas without passing through the liquid phase. Key differences include the intermediate state presence in condensation and its absence in sublimation, as well as the energy dynamics: condensation releases latent heat while sublimation requires energy absorption. These processes differ significantly in thermodynamics and environmental conditions, influencing applications like weather phenomena and freeze-drying technology.
Scientific Principles Behind Each Process
Condensation involves the phase transition of a substance from gas to liquid when molecules lose energy and move closer together, influenced by temperature and pressure changes. Sublimation occurs when a solid transitions directly to gas without passing through the liquid phase, driven by a substance's vapor pressure exceeding atmospheric pressure at specific conditions. Both processes demonstrate fundamental thermodynamic principles, including energy exchange and molecular kinetics.
Examples of Condensation in Daily Life
Condensation occurs when water vapor transforms into liquid water, commonly seen as dew forming on grass in the early morning or water droplets appearing on the outside of a cold glass. Another example includes fogging on bathroom mirrors during hot showers, where warm, moist air meets a cooler surface. These instances illustrate condensation's role in daily life by visibly demonstrating the phase change from gas to liquid.
Examples of Sublimation in Daily Life
Sublimation occurs when a solid transitions directly into a gas without passing through the liquid phase, as seen in the transformation of dry ice (solid carbon dioxide) into carbon dioxide gas used in fog machines and refrigeration. Another common example is the gradual disappearance of mothballs, which are solid naphthalene or paradichlorobenzene, sublimating to repel insects. Freeze-dried foods also demonstrate sublimation, where ice within the food sublimates under low pressure, preserving texture and nutrients.
Applications and Uses in Industry
Condensation finds extensive use in industries such as power generation, where steam turbines rely on condensers to convert exhaust steam back to water, improving efficiency. Sublimation is crucial in manufacturing processes like freeze-drying pharmaceuticals and purifying heat-sensitive materials, preserving product integrity. Both phase transitions are integral in HVAC systems, chemical processing, and material science for optimized thermal management and product quality control.
Environmental Impact of Each Process
Condensation reduces atmospheric water vapor, influencing local humidity and temperature regulation, while sublimation directly shifts ice to vapor, bypassing liquid water and affecting snowpack stability and water availability. The environmental impact of condensation often supports plant growth and ecosystem balance by contributing to precipitation cycles, whereas sublimation can accelerate glacier and ice cap shrinkage, exacerbating climate change effects. Both processes are critical in global water cycles but differ in their roles in energy exchange and hydrological distribution.
Summary: Condensation vs. Sublimation
Condensation is the process where water vapor changes into liquid water, typically occurring when warm, moist air cools. Sublimation involves the direct transition of a substance from solid to gas without passing through the liquid phase, commonly observed in dry ice (solid CO2) turning into carbon dioxide gas. Both processes are phase changes driven by temperature and pressure variations but represent opposite transitions in the state of matter.
Condensation Infographic
 libterm.com
libterm.com