Polar regions exhibit extreme climatic conditions that shape unique ecosystems and influence global weather patterns. Understanding the impact of polar ice melt on sea levels and biodiversity is crucial for assessing future environmental changes. Explore the rest of the article to discover how these icy landscapes affect your planet's health and what actions can be taken.
Table of Comparison
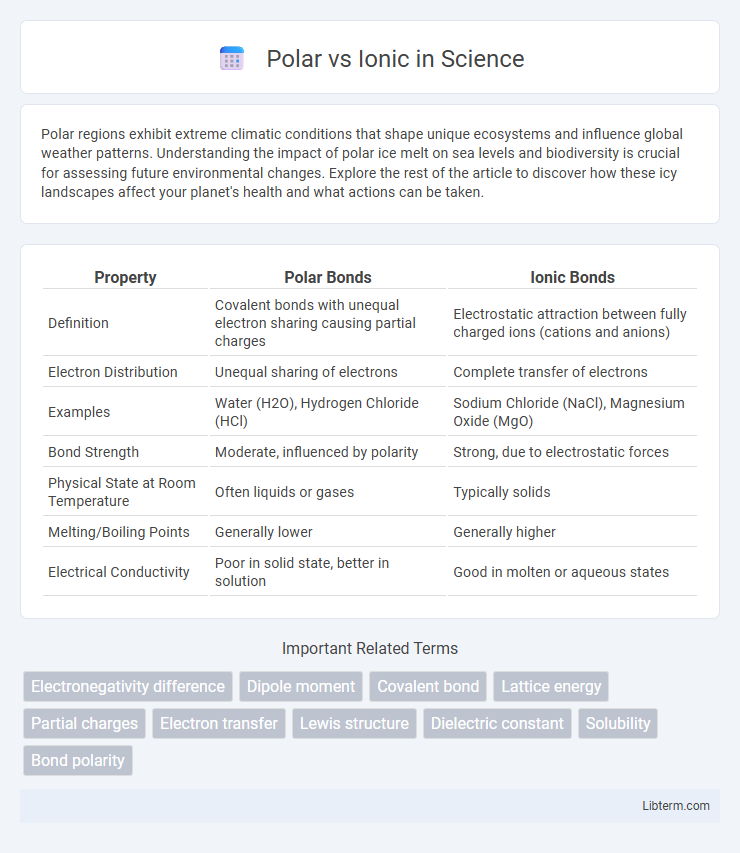
| Property | Polar Bonds | Ionic Bonds |
|---|---|---|
| Definition | Covalent bonds with unequal electron sharing causing partial charges | Electrostatic attraction between fully charged ions (cations and anions) |
| Electron Distribution | Unequal sharing of electrons | Complete transfer of electrons |
| Examples | Water (H2O), Hydrogen Chloride (HCl) | Sodium Chloride (NaCl), Magnesium Oxide (MgO) |
| Bond Strength | Moderate, influenced by polarity | Strong, due to electrostatic forces |
| Physical State at Room Temperature | Often liquids or gases | Typically solids |
| Melting/Boiling Points | Generally lower | Generally higher |
| Electrical Conductivity | Poor in solid state, better in solution | Good in molten or aqueous states |
Understanding Polar and Ionic Bonds
Polar bonds occur when electrons are shared unequally between atoms with different electronegativities, creating a dipole moment where one atom holds a partial negative charge, and the other a partial positive charge. Ionic bonds form through the complete transfer of electrons from a metal to a non-metal, resulting in positively charged cations and negatively charged anions attracted by strong electrostatic forces. Understanding the difference in electron distribution and bond strength between polar covalent and ionic bonds is key to predicting molecular behavior and properties.
Definition of Polar Bonds
Polar bonds occur when two atoms share electrons unequally due to differences in electronegativity, resulting in partial positive and negative charges within the molecule. The more electronegative atom attracts the bonding electrons more strongly, creating a dipole moment. This unequal electron distribution distinguishes polar covalent bonds from nonpolar covalent and ionic bonds.
Definition of Ionic Bonds
Ionic bonds form through the electrostatic attraction between positively charged cations and negatively charged anions, typically resulting from the complete transfer of electrons from one atom to another. This type of bond usually occurs between metals and nonmetals, creating a crystal lattice structure with high melting and boiling points. Ionic compounds exhibit strong polarity due to the significant difference in electronegativity between the bonded atoms.
Key Differences Between Polar and Ionic Compounds
Polar compounds exhibit uneven electron sharing between atoms with differing electronegativities, resulting in partial positive and negative charges within the molecule. Ionic compounds form through complete electron transfer from a metal to a nonmetal, creating positively charged cations and negatively charged anions held together by strong electrostatic forces. These differences influence properties such as melting points, solubility in water, and electrical conductivity, with ionic compounds generally having higher melting points and conductivity in molten or dissolved states compared to polar compounds.
How Polar Bonds Form
Polar bonds form when two atoms with different electronegativities share electrons unevenly, resulting in a partial positive charge on one atom and a partial negative charge on the other. This unequal electron distribution creates a dipole moment within the molecule, where the more electronegative atom attracts the bonding electrons closer. Such bonds are typically found in molecules like water (H2O), where oxygen is more electronegative than hydrogen, leading to distinct charge regions.
How Ionic Bonds Form
Ionic bonds form through the complete transfer of electrons from a metal atom to a non-metal atom, resulting in the creation of positively charged cations and negatively charged anions. This electron transfer occurs due to the significant difference in electronegativity between the two atoms, typically greater than 1.7 on the Pauling scale. The strong electrostatic attraction between these oppositely charged ions leads to the formation of a stable ionic compound.
Physical Properties: Polar vs Ionic
Polar compounds typically exhibit moderate melting and boiling points due to dipole-dipole interactions, while ionic compounds have significantly higher melting and boiling points as a result of strong electrostatic forces between ions. Polar substances tend to be soluble in polar solvents such as water, whereas ionic compounds dissolve readily in water due to ion-dipole interactions but are generally insoluble in nonpolar solvents. Electrical conductivity distinguishes ionic compounds in molten or aqueous states, where free ions enable conduction, unlike polar molecules that remain non-conductive in solid or liquid forms.
Real-World Examples of Polar and Ionic Compounds
Water (H2O) exemplifies a polar compound due to its uneven charge distribution and hydrogen bonding, enabling it to dissolve many substances and support life. Sodium chloride (NaCl) is a classic ionic compound formed from the transfer of electrons between sodium and chlorine atoms, resulting in a crystal lattice with high melting points and electrical conductivity when molten. Other real-world examples include hydrochloric acid (HCl) as a polar molecule used in industry, and calcium carbonate (CaCO3), an ionic compound found in limestone and seashells.
Importance of Polarity and Ionicity in Chemistry
Polarity and ionicity critically influence molecular interactions, solubility, and reactivity in chemical systems, determining how substances dissolve, bind, and participate in reactions. Polar molecules, with uneven charge distribution, enable hydrogen bonding and dipole-dipole interactions, essential for biological function and solvent behavior. Ionic compounds, composed of charged ions, exhibit high melting points and strong electrostatic forces, making them vital in inorganic chemistry and electrochemical applications.
Summary: Choosing Between Polar and Ionic
Selecting between polar and ionic compounds depends on their bonding characteristics and application needs. Polar compounds feature covalent bonds with unequal electron sharing, resulting in dipole moments suitable for solvents and biological interactions. Ionic compounds consist of electrostatically attracted ions, providing high melting points and electrical conductivity ideal for salts and electrolytes.
Polar Infographic
 libterm.com
libterm.com