Desiccated refers to something that has been thoroughly dried, often to preserve its quality or extend its shelf life. This process removes moisture, preventing the growth of bacteria and mold, making it common in food preservation and herb storage. Discover how desiccated products can benefit your daily life by reading the rest of the article.
Table of Comparison
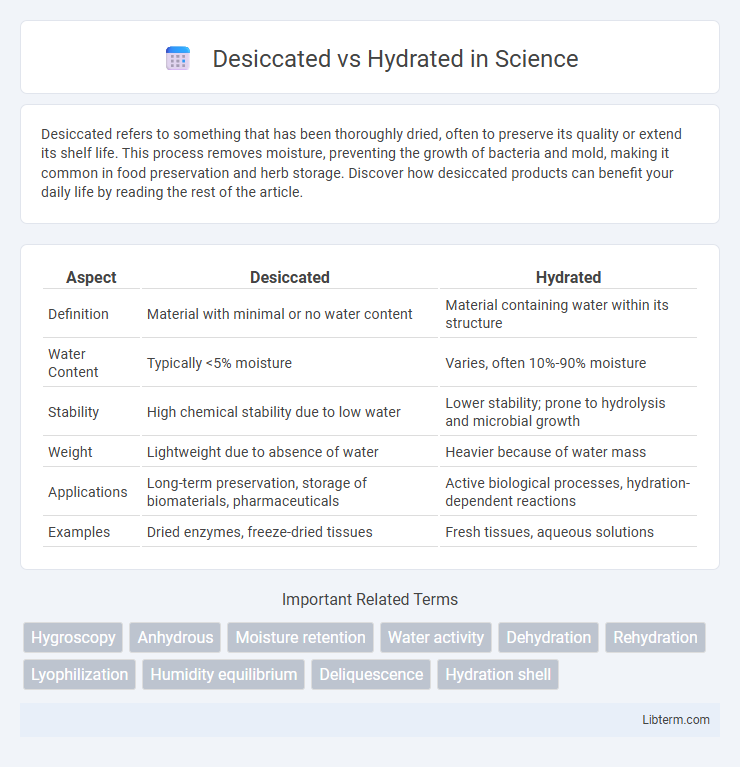
| Aspect | Desiccated | Hydrated |
|---|---|---|
| Definition | Material with minimal or no water content | Material containing water within its structure |
| Water Content | Typically <5% moisture | Varies, often 10%-90% moisture |
| Stability | High chemical stability due to low water | Lower stability; prone to hydrolysis and microbial growth |
| Weight | Lightweight due to absence of water | Heavier because of water mass |
| Applications | Long-term preservation, storage of biomaterials, pharmaceuticals | Active biological processes, hydration-dependent reactions |
| Examples | Dried enzymes, freeze-dried tissues | Fresh tissues, aqueous solutions |
Introduction to Desiccated vs Hydrated
Desiccated substances have been thoroughly dried to remove nearly all moisture, enhancing their shelf life and stability in various applications such as pharmaceuticals and food preservation. Hydrated substances, in contrast, contain water molecules integrated into their molecular structure, which can affect their physical and chemical properties, including solubility and reactivity. Understanding the distinction between desiccated and hydrated forms is critical for selecting appropriate materials based on their moisture content and functional requirements in scientific and industrial processes.
Understanding Desiccation and Hydration
Desiccation refers to the process of removing moisture from a substance, effectively preserving it by inhibiting microbial growth and chemical reactions. Hydration involves the absorption or combination of water molecules, essential for maintaining cellular function and biochemical processes. Understanding the balance between desiccation and hydration is crucial in fields such as food preservation, pharmaceuticals, and plant biology, where moisture control impacts quality and stability.
Scientific Principles Behind Desiccation
Desiccation involves the removal of water molecules from a substance, primarily through processes like dehydration or drying, which disrupts cellular biochemical reactions by limiting the availability of water as a solvent. This reduction in water content affects molecular mobility and enzyme activity, preserving materials by inhibiting microbial growth and chemical degradation. In contrast, hydrated substances retain water molecules, enabling normal biochemical functions and structural flexibility essential for biological processes.
Key Benefits of Desiccated Products
Desiccated products offer enhanced shelf stability by removing moisture, which inhibits microbial growth and prolongs freshness. Their concentrated nutrient content provides a potent source of vitamins, minerals, and enzymes, supporting better absorption and bioavailability. These features make desiccated products ideal for long-term storage and efficient nutrient delivery in dietary supplements and food applications.
Advantages of Hydrated Products
Hydrated products retain moisture, enhancing solubility and enabling faster rehydration in food and pharmaceutical applications. Their higher water content contributes to improved texture and flavor retention, offering a fresher sensory experience compared to desiccated counterparts. This moisture presence also helps preserve nutrient integrity, making hydrated products beneficial for maintaining bioavailability.
Applications in Food and Pharmaceuticals
Desiccated powders are widely used in food and pharmaceuticals for their extended shelf life and ease of storage, making them ideal for powdered supplements, spices, and dry active pharmaceutical ingredients (APIs). Hydrated forms retain moisture, which enhances solubility and bioavailability, crucial for liquid formulations, reconstituted medicines, and injectable drugs. Choosing between desiccated and hydrated forms depends on the specific requirements for stability, processing, and delivery in food products and pharmaceutical manufacturing.
Shelf Life and Storage Differences
Desiccated products have a significantly longer shelf life due to the removal of moisture, which inhibits microbial growth and slows down chemical degradation. Hydrated products require refrigeration or freezing to maintain freshness and prevent spoilage, resulting in a shorter shelf life compared to desiccated forms. Proper storage conditions for desiccated items typically involve cool, dry environments, while hydrated products demand controlled temperature and humidity levels to extend usability.
Impact on Nutritional Value
Desiccated foods undergo moisture removal, preserving nutrients by reducing enzymatic activity and microbial growth, but may lose some heat-sensitive vitamins like vitamin C during drying. Hydrated foods retain natural water content, maintaining higher levels of water-soluble vitamins and minerals, yet are more prone to nutrient degradation over time due to microbial activity. Understanding these differences is crucial for selecting foods that optimize nutrient retention in various dietary applications.
Choosing Between Desiccated and Hydrated
Choosing between desiccated and hydrated forms depends on the specific application requirements, such as shelf life, stability, and rehydration rate. Desiccated products offer longer storage and easier transportdue to reduced moisture, while hydrated forms provide immediate usability and enhanced bioavailability. Evaluating factors like environmental conditions, usage convenience, and processing costs ensures optimal selection for industrial, pharmaceutical, or food applications.
Conclusion: Making the Right Choice
Choosing between desiccated and hydrated forms depends on the specific application, environmental conditions, and desired shelf life. Desiccated materials offer enhanced stability and longer preservation by removing moisture, ideal for storage and transport. Hydrated forms retain water content, enabling immediate reactivity or bioavailability, suitable for processes requiring active interaction with moisture.
Desiccated Infographic
 libterm.com
libterm.com