Fermentation is a natural metabolic process where microorganisms like yeast and bacteria convert sugars into alcohol, gases, or acids, preserving food and enhancing flavor and texture. This anaerobic reaction plays a crucial role in producing diverse products such as bread, yogurt, wine, and sauerkraut. Discover how fermentation can benefit your health and culinary skills by exploring the rest of this article.
Table of Comparison
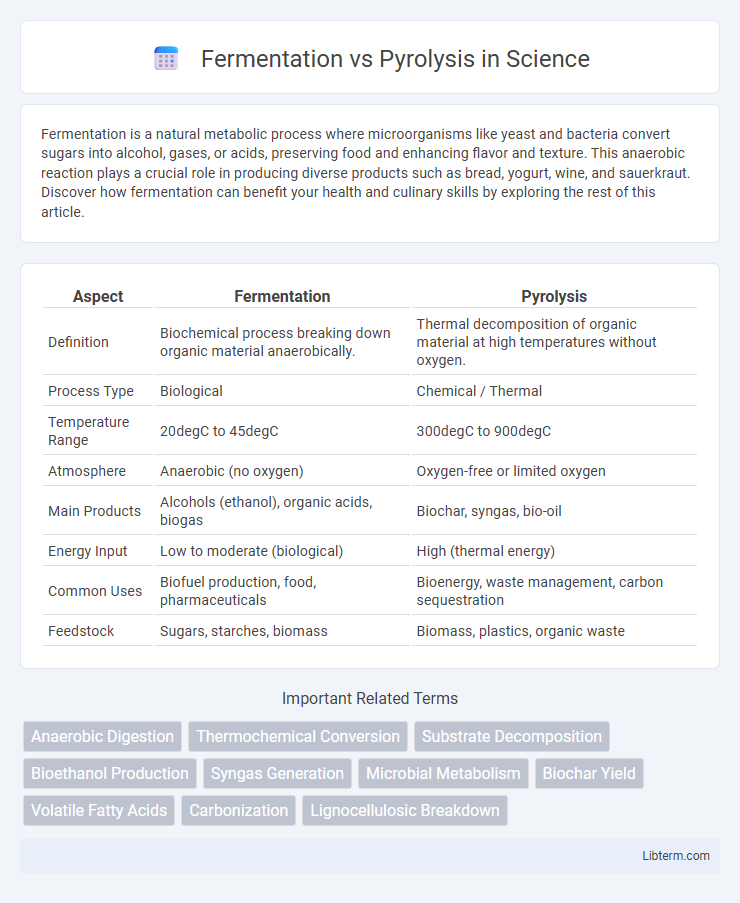
| Aspect | Fermentation | Pyrolysis |
|---|---|---|
| Definition | Biochemical process breaking down organic material anaerobically. | Thermal decomposition of organic material at high temperatures without oxygen. |
| Process Type | Biological | Chemical / Thermal |
| Temperature Range | 20degC to 45degC | 300degC to 900degC |
| Atmosphere | Anaerobic (no oxygen) | Oxygen-free or limited oxygen |
| Main Products | Alcohols (ethanol), organic acids, biogas | Biochar, syngas, bio-oil |
| Energy Input | Low to moderate (biological) | High (thermal energy) |
| Common Uses | Biofuel production, food, pharmaceuticals | Bioenergy, waste management, carbon sequestration |
| Feedstock | Sugars, starches, biomass | Biomass, plastics, organic waste |
Introduction to Fermentation and Pyrolysis
Fermentation is a biological process where microorganisms like bacteria and yeast convert organic substrates, primarily sugars, into alcohol, gases, or acids under anaerobic conditions. Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures in the absence of oxygen, producing biochar, bio-oil, and syngas. Both processes are integral to renewable energy production, with fermentation commonly used in bioethanol production and pyrolysis leveraged for biochar and synthetic fuel generation.
Defining Fermentation: Biological Processes
Fermentation is a biological process where microorganisms such as bacteria, yeast, or fungi convert organic substrates like sugars into simpler compounds, producing energy in anaerobic conditions. This metabolism leads to the creation of products such as ethanol, lactic acid, and carbon dioxide, widely used in food, beverages, and biofuel industries. Unlike pyrolysis, fermentation operates at low temperatures and relies on enzymatic reactions to break down biomass without combustion or thermal degradation.
Understanding Pyrolysis: Thermal Decomposition
Pyrolysis is a process of thermal decomposition that breaks down organic materials at elevated temperatures in the absence of oxygen, resulting in the production of char, bio-oil, and syngas. This method contrasts with fermentation, which relies on microbial activity under anaerobic conditions to convert biomass into bioethanol or biogas. Pyrolysis offers a faster and more versatile conversion of biomass into valuable fuels and chemicals, making it a key technology in sustainable energy production.
Key Differences Between Fermentation and Pyrolysis
Fermentation converts organic materials into energy-rich compounds like ethanol or methane using microorganisms under anaerobic conditions, whereas pyrolysis thermally decomposes biomass at high temperatures in the absence of oxygen to produce bio-oil, syngas, and char. Fermentation typically operates at lower temperatures (around 30-70degC) and is suitable for wet feedstocks like sugars and starches, while pyrolysis requires temperatures ranging from 300-700degC and treats dry biomass such as wood or agricultural residues. Key differences include reaction type (biochemical vs. thermochemical), temperature range, product types, and feedstock moisture content.
Applications of Fermentation in Industry
Fermentation plays a crucial role in industries such as food and beverage production, pharmaceuticals, and biofuels by converting sugars into valuable products like ethanol, antibiotics, and organic acids. Industrial fermentation processes utilize microorganisms like yeast and bacteria to produce beer, wine, yogurt, and bioplastics at scale. This biotechnological application supports sustainable manufacturing and contributes to reducing reliance on fossil fuels through bioethanol production.
Industrial Uses of Pyrolysis
Pyrolysis plays a crucial role in industrial waste management by converting biomass, plastics, and rubber into valuable products like bio-oil, syngas, and char. Its ability to efficiently process heterogeneous feedstocks at high temperatures without oxygen makes it ideal for sustainable energy production and chemical manufacturing. Industrial sectors leverage pyrolysis for producing renewable fuels, carbon black, and activated carbon, which are essential in energy, manufacturing, and environmental applications.
Energy Efficiency: Fermentation vs Pyrolysis
Fermentation typically operates at lower temperatures and converts biomass into biofuels like ethanol with moderate energy efficiency, often around 30-40% of the feedstock's energy content. Pyrolysis involves high-temperature thermal decomposition of organic material in the absence of oxygen, achieving energy efficiencies close to 60-70% by producing bio-oil, syngas, and char. The higher energy recovery in pyrolysis is due to its ability to capture multiple energy-rich products, whereas fermentation is limited to specific biochemical pathways that yield fewer energy-dense outputs.
Environmental Impacts: A Comparative Analysis
Fermentation produces lower greenhouse gas emissions by converting organic waste into biofuels and bioproducts with minimal toxic byproducts, promoting sustainable waste management and reducing reliance on fossil fuels. Pyrolysis involves thermal decomposition of organic materials at high temperatures, generating biochar, bio-oil, and syngas, but can release hazardous pollutants if not properly controlled, though biochar application enhances soil carbon sequestration. Comparing environmental impacts, fermentation offers cleaner energy conversion with fewer air pollutants, while pyrolysis contributes to carbon capture yet requires stringent emission controls to mitigate its potential environmental risks.
Economic Considerations: Cost and Scalability
Fermentation processes generally require lower capital investment and operate at moderate temperatures, making them cost-effective for small- to medium-scale biomass conversion, but they often face scalability challenges due to slower reaction rates and substrate specificity. Pyrolysis involves higher initial costs related to specialized high-temperature reactors and energy input, yet it offers greater scalability potential for diverse feedstocks and rapid conversion rates, which can reduce per-unit processing costs at large scales. Economic considerations must weigh fermentation's lower operational risks and simpler technology against pyrolysis's higher throughput and ability to produce versatile bio-oils and syngas for broader commercial applications.
Future Trends and Innovations in Fermentation and Pyrolysis
Future trends in fermentation emphasize genetic engineering advancements and synthetic biology to enhance microbial efficiency for biofuel and biochemical production, reducing costs and environmental impact. Innovations in pyrolysis focus on improving reactor designs and catalyst development to optimize biochar yield and syngas quality for renewable energy and soil amendment applications. Integration of AI and machine learning accelerates process optimization in both fermentation and pyrolysis, driving scalable, sustainable bio-based industries.
Fermentation Infographic
 libterm.com
libterm.com