Van der Waals forces are weak intermolecular attractions crucial for molecular interactions and biological processes. These forces influence phenomena such as adhesion, cohesion, and the behavior of gases and liquids. Discover more about how Van der Waals forces impact your everyday world in the rest of this article.
Table of Comparison
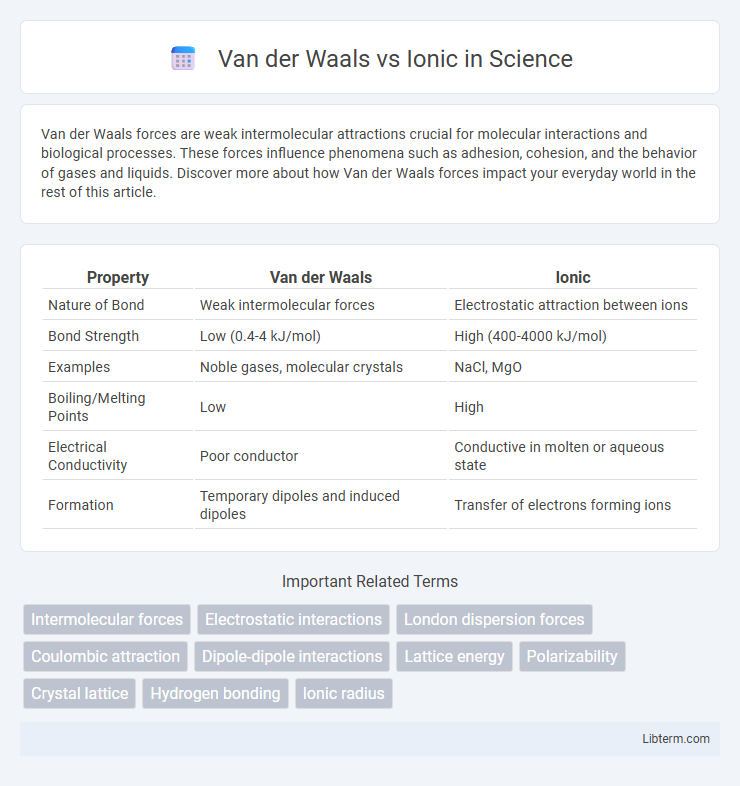
| Property | Van der Waals | Ionic |
|---|---|---|
| Nature of Bond | Weak intermolecular forces | Electrostatic attraction between ions |
| Bond Strength | Low (0.4-4 kJ/mol) | High (400-4000 kJ/mol) |
| Examples | Noble gases, molecular crystals | NaCl, MgO |
| Boiling/Melting Points | Low | High |
| Electrical Conductivity | Poor conductor | Conductive in molten or aqueous state |
| Formation | Temporary dipoles and induced dipoles | Transfer of electrons forming ions |
Introduction to Van der Waals and Ionic Interactions
Van der Waals interactions are weak, non-covalent forces arising from temporary dipoles in molecules, essential in molecular recognition and biological systems. Ionic interactions involve the electrostatic attraction between oppositely charged ions, forming strong bonds critical in salts and many inorganic compounds. Understanding the contrast between the transient, weak Van der Waals forces and the stronger, permanent ionic bonds is fundamental in chemistry and material science.
Defining Van der Waals Forces
Van der Waals forces are weak intermolecular interactions arising from temporary dipoles induced in atoms or molecules due to electron cloud fluctuations. These forces differ from ionic bonds, which involve the electrostatic attraction between positively and negatively charged ions formed through electron transfer. Van der Waals forces are critical in determining physical properties like boiling points and solubility in nonpolar substances, whereas ionic bonds create strong, stable crystal lattices in ionic compounds.
Understanding Ionic Bonds
Ionic bonds form through the electrostatic attraction between positively charged cations and negatively charged anions, typically between metals and nonmetals, resulting in a strong, stable lattice structure. Unlike the weak, distance-dependent Van der Waals forces found in molecular interactions, ionic bonds create high melting and boiling points due to their significant bond energy. Understanding ionic bonds is essential for explaining properties such as electrical conductivity in molten or aqueous states and the formation of crystalline solids like sodium chloride.
Key Differences: Van der Waals vs. Ionic Bonds
Van der Waals forces are weak intermolecular attractions caused by temporary dipoles, primarily found in nonpolar molecules, whereas ionic bonds involve the electrostatic attraction between oppositely charged ions, forming strong chemical bonds. Unlike ionic bonds that create high melting and boiling points due to lattice structures, Van der Waals forces result in low melting and boiling points since they only affect physical states. Ionic compounds are typically soluble in water and conduct electricity when molten or dissolved, while substances held by Van der Waals forces are generally insoluble and non-conductive.
Strength and Stability Comparison
Van der Waals forces are weak intermolecular attractions resulting from temporary dipoles, making them significantly less strong and less stable compared to ionic bonds. Ionic bonds arise from electrostatic attraction between oppositely charged ions, leading to high strength and enhanced stability in ionic compounds. The energy required to break ionic bonds is substantially greater than that needed to overcome Van der Waals interactions, reinforcing the superior durability of ionic structures.
Occurrence in Nature and Everyday Life
Van der Waals forces commonly occur in molecular solids like noble gases, organic compounds, and biological macromolecules, influencing processes such as protein folding and the adhesion of gecko feet to surfaces. Ionic bonds are prevalent in salts like sodium chloride and minerals such as halite and calcite, playing a critical role in the structure of ionic crystals and the conductivity of electrolytes in solutions. These interactions dictate the physical properties and stability of various natural and everyday materials.
Role in Chemical and Biological Systems
Van der Waals forces play a crucial role in stabilizing molecular structures and facilitating weak, reversible interactions in biological systems, such as protein folding and cell membrane integrity. Ionic bonds provide stronger, more permanent interactions essential for maintaining the structural framework of compounds like salts and stabilizing enzyme-substrate complexes. Both bond types contribute to molecular recognition, signaling, and structural organization in chemical and biological processes.
Impact on Material Properties
Van der Waals forces contribute to materials with low melting points, softness, and flexibility due to their weak intermolecular interactions, often observed in molecular solids like graphite and noble gases. Ionic bonds create materials with high melting points, hardness, and brittleness because of strong electrostatic attraction between oppositely charged ions, typical of compounds like sodium chloride and magnesium oxide. These fundamental differences impact electrical conductivity, solubility, and mechanical strength in applications ranging from semiconductors to ceramics.
Advantages and Limitations of Each Bond Type
Van der Waals forces offer advantages in flexibility and weak intermolecular attraction, making them crucial in biological systems and molecular recognition, yet their limitation lies in low bond strength and easy disruption by temperature changes. Ionic bonds provide significant advantages in forming strong, stable compounds with high melting points and excellent electrical conductivity in molten or aqueous states, though their brittleness and susceptibility to dissolution in polar solvents are key limitations. Understanding these properties aids in material selection for pharmaceuticals, electronics, and nanotechnology applications.
Summary Table: Van der Waals vs. Ionic Bonds
Van der Waals bonds are weak, non-covalent interactions primarily caused by transient dipoles in molecules, resulting in low melting points and poor electrical conductivity. Ionic bonds form from the electrostatic attraction between positively and negatively charged ions, resulting in high melting points and strong electrical conductivity in molten or aqueous states. The summary table highlights key distinctions: bond strength, type of interaction, typical physical properties, and common examples, providing a clear comparison between Van der Waals forces and ionic bonds.
Van der Waals Infographic
 libterm.com
libterm.com