Electrodes are critical components in electrical circuits, serving as the interface between electronic devices and conductive media to enable efficient current flow. Their material composition and design directly impact conductivity, durability, and performance across applications such as batteries, sensors, and welding. Discover how choosing the right electrode can enhance your device's functionality and longevity by reading the rest of the article.
Table of Comparison
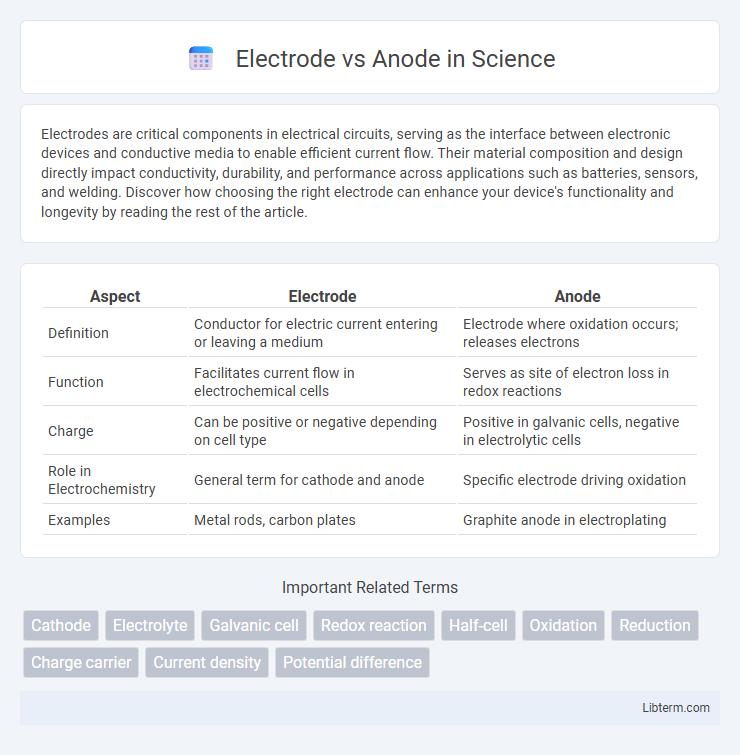
| Aspect | Electrode | Anode |
|---|---|---|
| Definition | Conductor for electric current entering or leaving a medium | Electrode where oxidation occurs; releases electrons |
| Function | Facilitates current flow in electrochemical cells | Serves as site of electron loss in redox reactions |
| Charge | Can be positive or negative depending on cell type | Positive in galvanic cells, negative in electrolytic cells |
| Role in Electrochemistry | General term for cathode and anode | Specific electrode driving oxidation |
| Examples | Metal rods, carbon plates | Graphite anode in electroplating |
Introduction to Electrodes and Anodes
Electrodes serve as conductors through which electric current enters or leaves a medium, playing a critical role in electrochemical cells. Anodes are a specific type of electrode where oxidation reactions occur, releasing electrons to the external circuit. Understanding the distinction between electrodes and anodes is essential for applications in batteries, electrolysis, and corrosion prevention.
Defining Electrode: A Broad Overview
An electrode is a conductive material that serves as the interface for electrical current to enter or leave a medium, commonly used in electrochemical cells and electronic devices. It encompasses both anodes and cathodes, which are specific types of electrodes defined by their roles in oxidation and reduction reactions, respectively. The broad definition of an electrode highlights its critical function in facilitating electron flow and enabling chemical transformations across various technological applications.
What is an Anode?
An anode is the electrode where oxidation occurs, releasing electrons to the external circuit in electrochemical cells. It serves as the positive terminal in electrolytic cells and the negative terminal in galvanic cells. Understanding its role is essential in applications like batteries, corrosion prevention, and electroplating.
Key Differences: Electrode vs Anode
The electrode is a general term for a conductor through which electric current enters or leaves a medium, while the anode specifically refers to the electrode where oxidation occurs and current flows into the device. In electrochemical cells, the anode is the negative terminal during discharge in galvanic cells but becomes the positive terminal in electrolytic cells. Understanding the distinct roles of anode and electrode is crucial for applications in batteries, electroplating, and corrosion prevention.
Roles of Electrodes in Electrochemical Cells
In electrochemical cells, electrodes serve as crucial sites for oxidation and reduction reactions, with the anode facilitating oxidation by releasing electrons and the cathode supporting reduction by gaining electrons. The anode acts as the electron source, driving the flow of current through the external circuit, while the cathode acts as the electron sink. These roles are essential in processes like galvanic cells and electrolytic cells, where electron transfer enables chemical energy conversion or electrolysis.
The Function of Anodes in Circuits
Anodes serve as the positive terminals in electrical circuits, facilitating the flow of current by attracting electrons toward themselves. In devices like diodes and electrolytic cells, anodes enable oxidation reactions by supplying electrons to the external circuit. Their function is crucial for directing current flow and ensuring proper operation of various electronic components.
Types of Electrodes and Anodes
Electrodes are conductive materials that facilitate electron transfer in electrochemical cells, categorized into types such as working electrodes, reference electrodes, and auxiliary electrodes, each serving distinct functions in processes like electrolysis and battery operation. Anodes, a specific type of electrode, serve as the site of oxidation where electrons are released, found in various forms including sacrificial anodes in corrosion protection systems, inert anodes in electrolytic cells, and fuel cell anodes designed for efficient electrochemical reactions. Understanding the material composition, such as graphite or platinum for electrodes and zinc or magnesium for sacrificial anodes, is crucial for optimizing performance in applications ranging from galvanic cells to industrial electroplating.
Common Applications: Where Are They Used?
Electrodes are essential components in devices like batteries, fuel cells, and electrochemical sensors, serving as the interface for electron transfer in various energy storage and conversion systems. Anodes, specifically, are widely used in applications such as lithium-ion batteries, electroplating, and corrosion protection, where they function as the site of oxidation reactions. Both components are critical in industrial processes, medical devices like EEG and ECG, and environmental technologies including water treatment and electrolysis.
Electrode and Anode Materials
Electrode materials vary widely depending on their application, with common choices including graphite, platinum, and carbon composites for increased conductivity and corrosion resistance. Anodes, specifically, are often made from materials like zinc, aluminum, or lead dioxide, chosen for their ability to undergo oxidation reactions efficiently in batteries or electrochemical cells. Understanding the material properties such as electrical conductivity, chemical stability, and cost is crucial for optimizing electrode and anode performance in energy storage and industrial processes.
Anode and Electrode Maintenance and Lifespan
Anodes, as the critical components in electrochemical cells, require regular maintenance to prevent corrosion buildup and ensure efficient current flow, directly impacting the cell's lifespan. Electrode maintenance involves cleaning and inspecting both anodes and cathodes to avoid surface contamination that can degrade performance and reduce durability. Proper care, such as timely replacement and application of protective coatings, significantly extends the operational life of electrodes in various industrial and electroplating applications.
Electrode Infographic
 libterm.com
libterm.com