Exothermic reactions release energy, usually in the form of heat, making the surroundings warmer. This energy release occurs when the total energy of the products is lower than that of the reactants, resulting in a net output of heat. Explore the rest of the article to understand how exothermic processes impact various scientific and industrial applications.
Table of Comparison
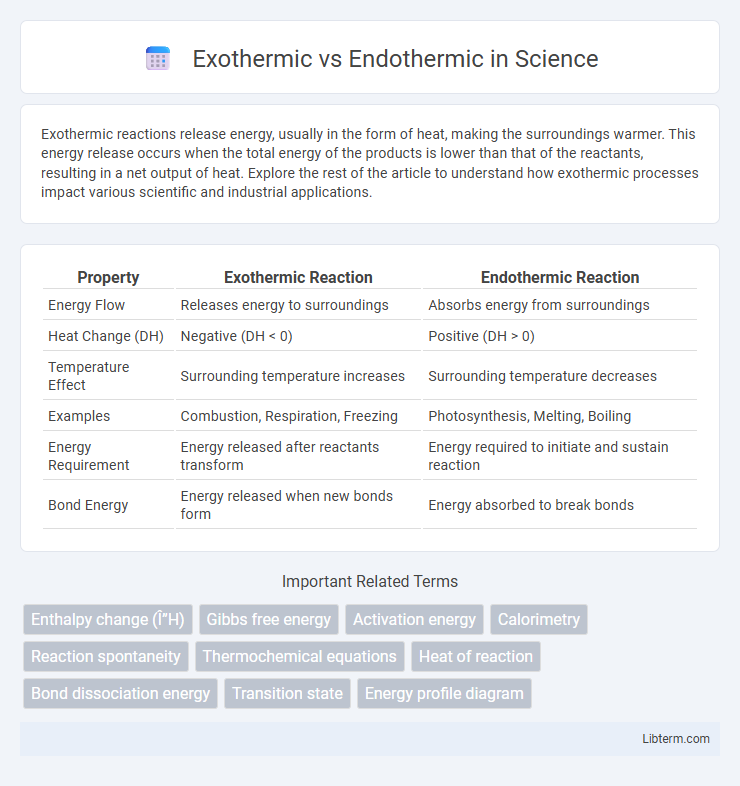
| Property | Exothermic Reaction | Endothermic Reaction |
|---|---|---|
| Energy Flow | Releases energy to surroundings | Absorbs energy from surroundings |
| Heat Change (DH) | Negative (DH < 0) | Positive (DH > 0) |
| Temperature Effect | Surrounding temperature increases | Surrounding temperature decreases |
| Examples | Combustion, Respiration, Freezing | Photosynthesis, Melting, Boiling |
| Energy Requirement | Energy released after reactants transform | Energy required to initiate and sustain reaction |
| Bond Energy | Energy released when new bonds form | Energy absorbed to break bonds |
Understanding Exothermic and Endothermic Reactions
Exothermic reactions release energy by transferring heat to the surroundings, causing an increase in temperature, commonly seen in combustion and respiration processes. Endothermic reactions absorb energy from the environment, resulting in a temperature drop, exemplified by photosynthesis and evaporative cooling. Understanding the energy flow in exothermic and endothermic reactions is essential for controlling chemical processes and optimizing energy efficiency in industrial and biological systems.
Key Differences Between Exothermic and Endothermic Processes
Exothermic processes release energy, usually in the form of heat, causing the surrounding temperature to increase, while endothermic processes absorb energy, leading to a temperature decrease in the surroundings. Key differences include energy flow direction, with exothermic reactions transferring energy from the system to the environment and endothermic reactions requiring energy input to proceed. Examples of exothermic processes are combustion and condensation, whereas melting and photosynthesis are common endothermic processes.
Energy Flow in Chemical Reactions
Exothermic reactions release energy to the surroundings, typically in the form of heat, resulting in a temperature increase of the environment. Endothermic reactions absorb energy from the surroundings, causing a temperature decrease as energy is taken in to break chemical bonds. The energy flow in chemical reactions reflects the difference in enthalpy between reactants and products, with exothermic processes having a negative enthalpy change (DH < 0) and endothermic processes having a positive enthalpy change (DH > 0).
Real-Life Examples of Exothermic Reactions
Exothermic reactions release energy, commonly observed in combustion processes such as burning wood or gasoline, which emit heat and light. Another example includes the respiration of glucose in cells, where chemical energy is converted into usable ATP along with heat production. Additionally, the formation of ice from water is an exothermic phase change, releasing latent heat into the surroundings.
Common Endothermic Reactions in Daily Life
Common endothermic reactions in daily life include photosynthesis, where plants absorb sunlight to convert carbon dioxide and water into glucose and oxygen. The melting of ice absorbs heat from the surroundings, causing temperature drops. Evaporation of sweat is another everyday endothermic process that cools the body by absorbing heat.
Temperature Changes and Reaction Dynamics
Exothermic reactions release heat, causing the surrounding temperature to rise as reactants transform into products with lower energy states. Endothermic reactions absorb heat, leading to a temperature drop in the environment while reactants gain energy to form higher-energy products. The reaction dynamics differ significantly; exothermic processes often proceed spontaneously due to energy release, whereas endothermic reactions require continuous energy input to maintain progress.
Enthalpy Changes: Exothermic vs Endothermic
Exothermic reactions release heat energy, resulting in a negative enthalpy change (DH < 0) as the system loses enthalpy to the surroundings. Endothermic reactions absorb heat energy, producing a positive enthalpy change (DH > 0) since the system gains enthalpy from the surroundings. The magnitude and sign of enthalpy change are key indicators in determining whether a chemical process is exothermic or endothermic.
Practical Applications in Industry and Technology
Exothermic reactions are crucial in industries like metallurgy and power generation, where the release of heat is harnessed for processes such as steel manufacturing and combustion engines. Endothermic reactions find applications in refrigeration, chemical synthesis, and materials processing, requiring heat absorption to drive reactions like photosynthesis in bioengineering and ammonia production in the Haber process. Understanding the thermal dynamics of these reactions enhances energy efficiency and process control in manufacturing and environmental technologies.
Factors Affecting Reaction Heat Exchange
Temperature, pressure, and the nature of reactants significantly influence whether a reaction is exothermic or endothermic. Bond energy differences determine heat release or absorption, where stronger bonds formed in products result in exothermic reactions, and weaker bonds formed cause endothermic processes. Catalysts and the physical state of reactants also alter activation energy, affecting heat exchange dynamics during the reaction.
Safety Considerations for Exothermic and Endothermic Reactions
Exothermic reactions release heat, posing risks such as burns, fires, and explosions, thus requiring proper ventilation, heat dissipation measures, and use of protective equipment like gloves and goggles. Endothermic reactions absorb heat, which can lead to dangerously low temperatures and potential freezing hazards, necessitating temperature monitoring and insulation to prevent thermal shock or equipment damage. Both types demand strict adherence to safety protocols to manage energy changes and ensure safe handling in laboratory or industrial settings.
Exothermic Infographic
 libterm.com
libterm.com