Apolar molecules lack a significant difference in electronegativity between their atoms, resulting in no permanent dipole moment and making them generally non-polar and hydrophobic. These molecules tend to dissolve well in non-polar solvents but poorly in polar solvents like water. Discover how understanding apolar characteristics can impact your approach by reading the rest of the article.
Table of Comparison
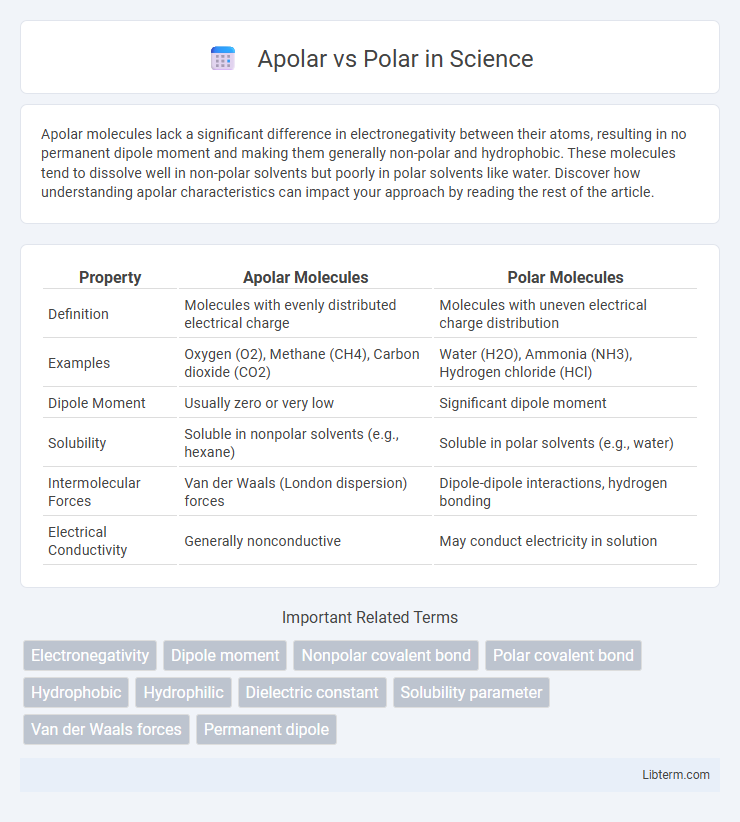
| Property | Apolar Molecules | Polar Molecules |
|---|---|---|
| Definition | Molecules with evenly distributed electrical charge | Molecules with uneven electrical charge distribution |
| Examples | Oxygen (O2), Methane (CH4), Carbon dioxide (CO2) | Water (H2O), Ammonia (NH3), Hydrogen chloride (HCl) |
| Dipole Moment | Usually zero or very low | Significant dipole moment |
| Solubility | Soluble in nonpolar solvents (e.g., hexane) | Soluble in polar solvents (e.g., water) |
| Intermolecular Forces | Van der Waals (London dispersion) forces | Dipole-dipole interactions, hydrogen bonding |
| Electrical Conductivity | Generally nonconductive | May conduct electricity in solution |
Understanding Polarity: Apolar vs Polar
Polarity in molecules is determined by the difference in electronegativity between atoms, with polar molecules exhibiting uneven electron distribution leading to partial positive and negative charges. Apolar (nonpolar) molecules have an even electron distribution due to similar electronegativity or symmetrical structures, resulting in no significant dipole moment. Understanding polarity is crucial for predicting solubility, intermolecular interactions, and chemical reactivity in various substances.
Defining Apolar and Polar Molecules
Apolar molecules are characterized by the equal distribution of electrical charge, resulting in no permanent dipole moment, typically found in nonpolar covalent bonds such as in oxygen (O2) or nitrogen (N2) molecules. Polar molecules possess an uneven distribution of electron density that creates partial positive and negative charges, exemplified by water (H2O) and hydrogen chloride (HCl). The polarity of a molecule directly influences its solubility, boiling point, and intermolecular interactions in chemical and biological processes.
Chemical Bonding and Electronegativity Differences
Apolar bonds occur when atoms share electrons equally due to similar electronegativity values, resulting in nonpolar covalent bonds such as in diatomic molecules like O2 or N2. Polar bonds form when electronegativity differences between bonded atoms cause unequal electron sharing, creating partial positive and negative charges, as seen in molecules like H2O and HF. Electronegativity differences greater than 0.4 typically indicate polar covalent bonds, whereas differences close to zero correspond to apolar covalent bonds.
Structural Characteristics of Apolar Molecules
Apolar molecules consist primarily of nonpolar covalent bonds where electrons are shared equally between atoms, resulting in a symmetrical electron distribution. Their molecular structures lack significant electronegativity differences, causing these molecules to be electrically neutral with minimal dipole moments. Common examples include hydrocarbons like methane and ethane, which exhibit low polarity due to their symmetrical geometry and uniform charge distribution.
Structural Features of Polar Molecules
Polar molecules possess an uneven distribution of electron density due to differences in electronegativity between atoms, creating partial positive and negative charges within the molecule. The molecular geometry plays a crucial role in polarity, as asymmetrical shapes prevent the cancellation of individual bond dipoles, resulting in a net dipole moment. Functional groups such as hydroxyl (-OH) or carbonyl (C=O) contribute significantly to polarity by adding regions of high electron density that influence intermolecular interactions.
Physical Properties: Solubility, Melting, and Boiling Points
Apolar molecules exhibit low solubility in water but high solubility in nonpolar solvents due to weak intermolecular forces and lack of polarity. Polar molecules have higher melting and boiling points as strong dipole-dipole interactions and hydrogen bonding increase intermolecular attraction. The significant difference in solubility and phase transition temperatures between apolar and polar substances is crucial for applications in chemistry and material science.
Real-World Examples of Apolar and Polar Compounds
Water exemplifies polar compounds with its bent molecular shape and uneven charge distribution, enabling hydrogen bonding and high solubility in other polar substances. In contrast, hydrocarbons such as methane and benzene represent apolar compounds, characterized by their symmetric molecular structures and nonpolar covalent bonds, resulting in low solubility in water but high solubility in other apolar solvents like hexane. These differences in polarity critically influence their behavior in biological systems, industrial applications, and environmental processes.
Biological Significance of Polarity
Molecular polarity influences cellular interactions and membrane permeability in biological systems. Polar molecules, such as water and electrolytes, are essential for biochemical reactions and signal transduction due to their ability to form hydrogen bonds. Apolar molecules, including lipids, contribute to the structural integrity of cell membranes by creating hydrophobic barriers critical for compartmentalization and selective transport.
Polarity and Its Role in Solvent Selection
Polarity is a critical factor in solvent selection, as polar solvents have uneven charge distribution allowing them to dissolve ionic and other polar substances effectively. Apolar solvents, with their non-polar molecular structures, are better suited for dissolving non-polar compounds such as oils and fats due to their lack of significant charge separation. Understanding solvent polarity enables chemists to predict solubility behaviors and optimize reactions by matching solvent polarity with the target solute's polarity.
Key Differences Between Apolar and Polar Molecules
Apolar molecules have nonpolar covalent bonds with an equal or nearly equal distribution of electron density, resulting in no permanent dipole moment, whereas polar molecules possess polar covalent bonds with unequal electron sharing, leading to a permanent dipole moment. Apolar molecules are typically hydrophobic and do not dissolve well in water, while polar molecules are hydrophilic and readily dissolve in aqueous environments. The key differences between apolar and polar molecules significantly affect their physical properties, solubility, and intermolecular interactions such as hydrogen bonding and dipole-dipole forces.
Apolar Infographic
 libterm.com
libterm.com