Ionic is a powerful open-source framework for building cross-platform mobile applications using web technologies like HTML, CSS, and JavaScript. It enables developers to create high-performance apps with a native look and feel for both iOS and Android from a single codebase. Discover how Ionic can transform your app development process by exploring the rest of this article.
Table of Comparison
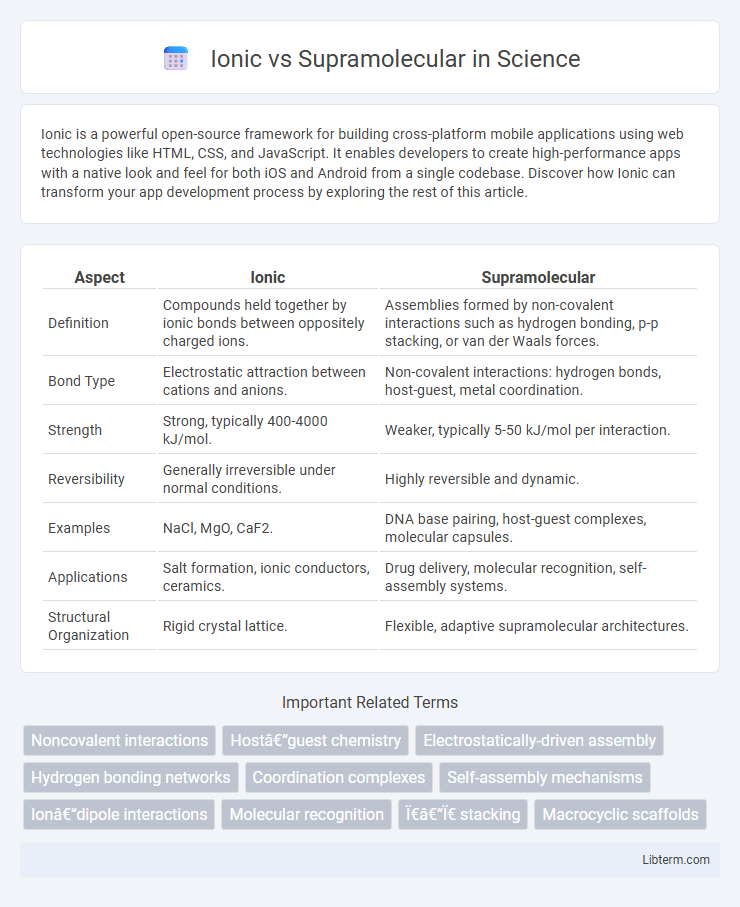
| Aspect | Ionic | Supramolecular |
|---|---|---|
| Definition | Compounds held together by ionic bonds between oppositely charged ions. | Assemblies formed by non-covalent interactions such as hydrogen bonding, p-p stacking, or van der Waals forces. |
| Bond Type | Electrostatic attraction between cations and anions. | Non-covalent interactions: hydrogen bonds, host-guest, metal coordination. |
| Strength | Strong, typically 400-4000 kJ/mol. | Weaker, typically 5-50 kJ/mol per interaction. |
| Reversibility | Generally irreversible under normal conditions. | Highly reversible and dynamic. |
| Examples | NaCl, MgO, CaF2. | DNA base pairing, host-guest complexes, molecular capsules. |
| Applications | Salt formation, ionic conductors, ceramics. | Drug delivery, molecular recognition, self-assembly systems. |
| Structural Organization | Rigid crystal lattice. | Flexible, adaptive supramolecular architectures. |
Introduction: Ionic vs Supramolecular Interactions
Ionic interactions involve electrostatic attraction between oppositely charged ions, crucial for the stability of salts and many biomolecules. Supramolecular interactions encompass non-covalent forces such as hydrogen bonding, p-p stacking, and van der Waals forces, enabling reversible and dynamic assemblies. Understanding the differences between ionic and supramolecular interactions is essential for designing advanced materials and molecular systems with tailored properties.
Defining Ionic Bonds
Ionic bonds form through the electrostatic attraction between positively charged cations and negatively charged anions, resulting from the complete transfer of electrons between atoms. These bonds create strong, stable compounds with high melting points, such as sodium chloride. Unlike ionic bonds, supramolecular interactions involve non-covalent forces like hydrogen bonding, p-p stacking, and van der Waals interactions, enabling reversible and dynamic assembly of molecular structures.
Understanding Supramolecular Chemistry
Supramolecular chemistry explores the complex interactions between molecules through non-covalent bonds such as hydrogen bonding, van der Waals forces, and electrostatic interactions, contrasting with ionic chemistry which primarily relies on electrostatic attraction between charged ions. This field emphasizes molecular recognition, self-assembly, and stimuli-responsive systems, enabling the design of dynamic materials and advanced drug delivery mechanisms. Understanding these principles is crucial for developing supramolecular architectures that mimic biological systems and enable targeted functionalities beyond traditional ionic compounds.
Key Differences in Binding Mechanisms
Ionic interactions involve electrostatic attraction between positively and negatively charged ions, providing strong, directional binding with high specificity in salt bridges and ionic bonds. Supramolecular binding mechanisms rely on non-covalent forces such as hydrogen bonding, p-p interactions, van der Waals forces, and metal coordination, enabling reversible, dynamic assemblies that are highly tunable. The key difference lies in ionic bonds providing fixed, strong attachments, while supramolecular interactions allow for flexible, adaptive molecular recognition and self-assembly.
Structural Features and Stability
Ionic compounds are characterized by strong electrostatic forces between oppositely charged ions, leading to high melting points and significant lattice energy, which contribute to their structural rigidity and thermal stability. Supramolecular structures rely on non-covalent interactions such as hydrogen bonding, p-p stacking, and van der Waals forces, providing dynamic and reversible assembly with enhanced molecular recognition but typically lower thermal stability compared to ionic lattices. The balance between directional bonding in supramolecular systems and the strong ionic lattice results in differing mechanical properties and environmental responsiveness.
Applications in Material Science
Ionic bonds are widely used in material science for creating highly stable and conductive materials such as solid electrolytes in batteries and ionic liquids for chemical sensing. Supramolecular interactions, involving non-covalent forces like hydrogen bonding and pi-pi stacking, enable the design of self-healing materials, responsive hydrogels, and molecular recognition systems. Tailoring these interactions allows precise control over material properties, enhancing applications in nanotechnology, drug delivery, and smart coatings.
Role in Biological Systems
Ionic interactions play a critical role in biological systems by stabilizing protein structures, facilitating enzyme-substrate binding, and enabling nerve signal transmission through ion channels. Supramolecular chemistry governs the assembly of biomolecules via non-covalent forces such as hydrogen bonding, van der Waals forces, and hydrophobic interactions, essential for DNA base pairing, membrane formation, and molecular recognition. Understanding these interactions enhances drug design and the development of biomimetic materials by manipulating the specific and reversible nature of supramolecular assemblies versus the strong electrostatic forces of ionic bonds.
Advantages and Limitations
Ionic interactions offer strong electrostatic forces useful for creating stable materials with high conductivity but often suffer from poor selectivity and limited reversibility, impacting their adaptability in dynamic systems. Supramolecular chemistry provides highly selective, reversible, and stimuli-responsive assemblies driven by non-covalent interactions such as hydrogen bonding and p-p stacking, enabling advanced functional materials with tunable properties. However, supramolecular systems typically exhibit weaker bonding strength than ionic counterparts, which can limit mechanical robustness and long-term stability under harsh conditions.
Recent Advances and Research Trends
Recent advances in ionic materials highlight their tunable electrochemical properties, making them essential for energy storage and conversion applications, with research emphasizing ionic liquids and solid electrolytes. Supramolecular chemistry focuses on non-covalent interactions to design complex architectures with dynamic and responsive behaviors, advancing drug delivery systems and smart materials. Emerging trends in both fields include integrating ionic motifs into supramolecular assemblies to enhance functionality in sensors, catalysis, and nanotechnology.
Future Prospects and Innovations
Ionic materials continue to advance in energy storage and flexible electronics due to their high conductivity and tunable electrochemical properties. Supramolecular chemistry drives innovations in self-healing materials and smart drug delivery systems by leveraging reversible non-covalent interactions for dynamic functionality. Future prospects highlight the integration of ionic and supramolecular strategies to create hybrid systems with enhanced responsiveness, adaptability, and multifunctionality.
Ionic Infographic
 libterm.com
libterm.com