Synergism occurs when two or more elements combine to produce a result greater than the sum of their individual effects, enhancing overall performance and effectiveness. Understanding the principles of synergism can help you optimize collaborative efforts and achieve superior outcomes in various fields. Explore this article to discover how synergism can transform your approach to teamwork and productivity.
Table of Comparison
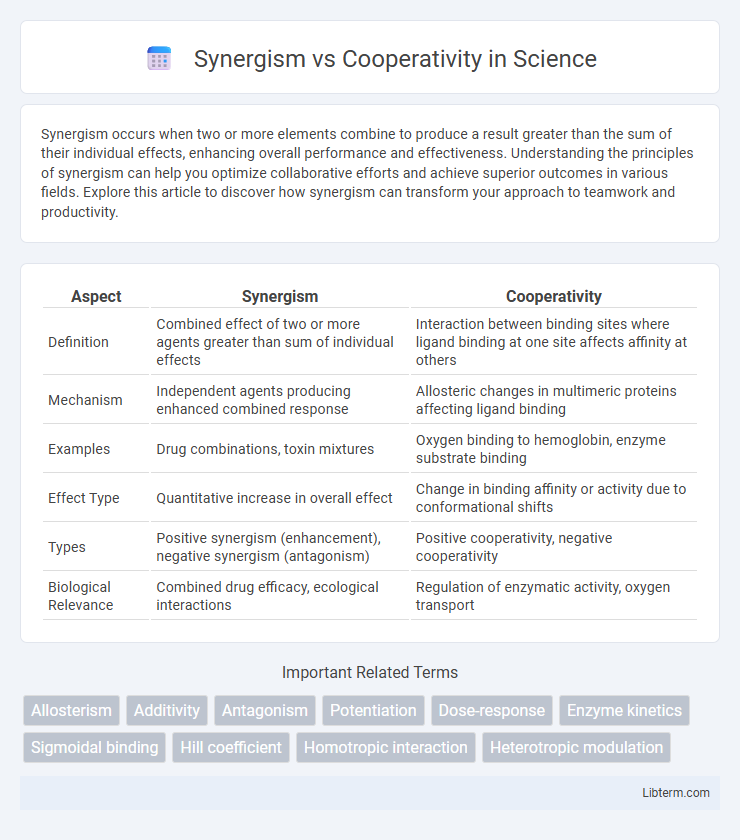
| Aspect | Synergism | Cooperativity |
|---|---|---|
| Definition | Combined effect of two or more agents greater than sum of individual effects | Interaction between binding sites where ligand binding at one site affects affinity at others |
| Mechanism | Independent agents producing enhanced combined response | Allosteric changes in multimeric proteins affecting ligand binding |
| Examples | Drug combinations, toxin mixtures | Oxygen binding to hemoglobin, enzyme substrate binding |
| Effect Type | Quantitative increase in overall effect | Change in binding affinity or activity due to conformational shifts |
| Types | Positive synergism (enhancement), negative synergism (antagonism) | Positive cooperativity, negative cooperativity |
| Biological Relevance | Combined drug efficacy, ecological interactions | Regulation of enzymatic activity, oxygen transport |
Introduction to Synergism and Cooperativity
Synergism and cooperativity both describe enhanced effects resulting from interactions between molecules or agents, but differ in mechanism and context. Synergism occurs when combined molecules produce a greater effect than the sum of their individual effects, often seen in pharmacology where drug combinations amplify therapeutic outcomes. Cooperativity refers to the binding phenomenon in proteins, such as hemoglobin, where the binding of one ligand influences the affinity of subsequent ligand bindings, exemplifying positive or negative modulation at the molecular level.
Defining Synergism: Key Concepts
Synergism describes the interaction of two or more agents or factors whose combined effect is greater than the sum of their individual effects, enhancing overall potency or efficiency in biological or chemical systems. Key concepts include the amplification of outcomes through complementary mechanisms and the non-linear increase in activity that cannot be explained by simple additive effects. Understanding synergism involves analyzing dose-response relationships and interaction indices to quantify the enhanced combined effect distinct from cooperativity.
Understanding Cooperativity in Biological Systems
Cooperativity in biological systems refers to the phenomenon where the binding of a ligand to one site on a macromolecule influences the binding affinity of additional sites, commonly observed in enzymes and receptors such as hemoglobin. This effect enhances the sensitivity and efficiency of biological responses, often modeled by the Hill coefficient to quantify the degree of cooperativity. Understanding cooperativity enables insights into allosteric regulation, signal transduction, and metabolic control mechanisms fundamental to cellular function.
Differences Between Synergism and Cooperativity
Synergism occurs when two or more agents produce an effect greater than the sum of their individual effects, often involving independent mechanisms, whereas cooperativity refers to the interaction within a single protein or enzyme complex where binding of one ligand affects the binding affinity of additional ligands. In synergism, the combined effect amplifies overall functionality through distinct pathways, while cooperativity relies on conformational changes within a molecule that modulate activity. This fundamental difference highlights synergism as a multi-agent phenomenon and cooperativity as an intrinsic molecular property.
Synergism in Drug Interactions and Therapeutics
Synergism in drug interactions occurs when combined drugs produce a therapeutic effect greater than the sum of their individual effects, enhancing efficacy and reducing required dosages. This phenomenon often results from complementary mechanisms of action, such as one drug enhancing the bioavailability or receptor sensitivity to another. Understanding synergism is crucial in designing combination therapies for diseases like cancer and infectious conditions, where maximizing therapeutic outcomes while minimizing toxicity is essential.
Cooperativity in Enzyme Kinetics and Protein Binding
Cooperativity in enzyme kinetics involves multiple binding sites where substrate binding at one site affects the affinity at other sites, often described by the Hill coefficient. This phenomenon enhances enzyme responsiveness to substrate concentration changes, important in allosteric enzymes like hemoglobin. Positive cooperativity facilitates substrate binding, while negative cooperativity reduces it, influencing metabolic regulation and protein function.
Mechanistic Insights: How Synergism and Cooperativity Work
Synergism occurs when combined effects of molecules produce an outcome greater than the sum of individual actions, often involving distinct binding sites or pathways that amplify the response. Cooperativity involves changes in binding affinity or activity at one site induced by ligand binding at another, frequently described by allosteric models like the Monod-Wyman-Changeux (MWC) model. Mechanistically, synergism enhances overall effect through complementary mechanisms, whereas cooperativity modulates the functional state of a protein complex to regulate binding dynamics and activity.
Measuring and Quantifying Synergism vs. Cooperativity
Measuring synergism involves quantifying the combined effect of two or more agents exceeding the sum of their individual effects, often using methods like isobologram analysis, combination index (CI), or Loewe additivity models. Cooperativity is quantified by assessing changes in ligand binding affinity at multiple sites within a single molecule, typically analyzed using Hill coefficients or binding curves to determine positive or negative cooperativity. Distinguishing synergism from cooperativity requires precise experimental designs and mathematical models that evaluate interactions between separate agents versus intramolecular binding site interactions.
Real-World Applications and Case Studies
Synergism in pharmacology enhances drug effectiveness by combining agents that produce a greater therapeutic effect together than individually, as seen in cancer treatment regimens using chemotherapy drug combinations like cyclophosphamide and doxorubicin. Cooperativity, often observed in enzymology, involves binding affinity changes upon ligand interaction, exemplified by hemoglobin's oxygen-binding dynamics that improve oxygen transport efficiency in mammals. Real-world applications include antibiotic synergy in treating resistant infections and cooperative enzyme mechanisms in industrial biocatalysis, highlighting their importance in drug development and biotechnology.
Summary and Future Perspectives
Synergism occurs when combined effects of molecules exceed the sum of individual actions, while cooperativity involves changes in binding affinity among multiple ligand sites on a single protein or complex. Future research aims to elucidate the molecular mechanisms underlying these interactions using advanced biophysical techniques and computational modeling to optimize drug design and therapeutic interventions. Understanding the distinct yet overlapping principles of synergism and cooperativity will enhance precision medicine strategies and multi-target drug development.
Synergism Infographic
 libterm.com
libterm.com