Open-chain compounds feature a linear or branched arrangement of atoms without forming rings, commonly found in organic chemistry. These structures influence reactivity, physical properties, and biological activity differently compared to cyclic compounds. Explore the article to understand open-chain compounds' significance and applications in various fields.
Table of Comparison
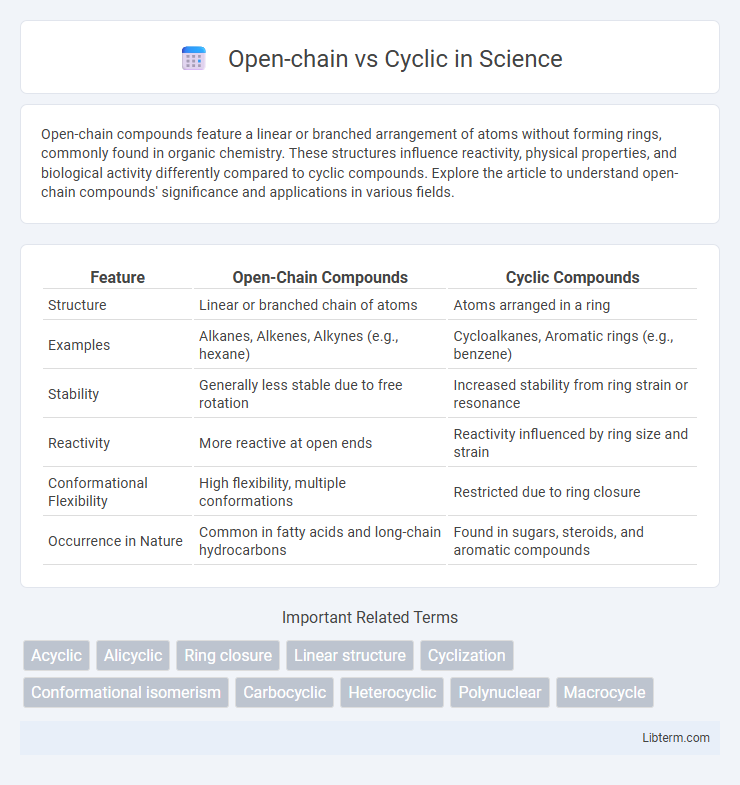
| Feature | Open-Chain Compounds | Cyclic Compounds |
|---|---|---|
| Structure | Linear or branched chain of atoms | Atoms arranged in a ring |
| Examples | Alkanes, Alkenes, Alkynes (e.g., hexane) | Cycloalkanes, Aromatic rings (e.g., benzene) |
| Stability | Generally less stable due to free rotation | Increased stability from ring strain or resonance |
| Reactivity | More reactive at open ends | Reactivity influenced by ring size and strain |
| Conformational Flexibility | High flexibility, multiple conformations | Restricted due to ring closure |
| Occurrence in Nature | Common in fatty acids and long-chain hydrocarbons | Found in sugars, steroids, and aromatic compounds |
Introduction to Open-chain and Cyclic Compounds
Open-chain compounds consist of carbon atoms connected in straight or branched chains without forming rings, commonly found in alkanes, alkenes, and alkynes, playing a fundamental role in organic chemistry due to their structural simplicity and reactivity. Cyclic compounds feature carbon atoms linked to form ring structures, which can be either aromatic, like benzene, or non-aromatic, such as cycloalkanes, imparting distinct chemical and physical properties compared to open-chain analogs. Understanding the differences in bonding, stability, and reactivity between open-chain and cyclic compounds is essential for exploring advanced topics in organic synthesis and molecular behavior.
Defining Open-chain Structures
Open-chain structures in organic chemistry consist of acyclic molecules with carbon atoms connected in unbranched or branched chains without forming rings. These structures include straight-chain alkanes, alkenes, and alkynes, where the carbon atoms have free ends and do not cyclize. Understanding open-chain molecules is essential for distinguishing their physical and chemical properties from cyclic compounds, which have ring structures.
Defining Cyclic Compounds
Cyclic compounds are chemical structures where atoms are connected in a closed ring or loop, creating a distinct, stable formation that contrasts with open-chain or acyclic compounds. These rings can vary in size, typically containing three or more atoms, and significantly influence the compound's chemical properties and reactivity. Understanding the distinction between cyclic and open-chain compounds is essential in organic chemistry because cyclic structures often exhibit unique stability, electronic distribution, and spatial arrangements.
Structural Differences: Open-chain vs Cyclic
Open-chain compounds consist of atoms connected in a linear or branched sequence without forming a ring, whereas cyclic compounds have atoms connected in a closed loop or ring structure. The structural differences impact their chemical reactivity and physical properties; for instance, cyclic structures often exhibit ring strain and unique stereochemistry not present in open-chain analogs. These variations influence stability, boiling points, and reactivity patterns crucial in organic synthesis and molecular design.
Examples of Open-chain Compounds
Open-chain compounds, also known as acyclic compounds, consist of carbon atoms connected in linear or branched chains without forming rings. Examples include ethanol (C2H5OH), acetone (C3H6O), and butane (C4H10), which are commonly found in organic chemistry. These compounds contrast with cyclic compounds like benzene and cyclohexane, where carbon atoms form closed rings.
Examples of Cyclic Compounds
Cyclic compounds include important examples such as benzene, cyclohexane, and pyridine, which feature ring structures that influence their chemical properties and reactivity. Benzene, a six-membered aromatic ring with alternating double bonds, serves as a fundamental building block in organic chemistry and industrial applications. Cyclohexane, a saturated six-carbon ring, is widely used as a solvent and intermediate in chemical synthesis, while heterocyclic compounds like pyridine incorporate nitrogen atoms, playing crucial roles in pharmaceuticals and agrochemicals.
Chemical Properties Comparison
Open-chain compounds exhibit greater flexibility and reactivity due to their free terminal ends, enabling easier participation in nucleophilic and electrophilic reactions. Cyclic compounds possess ring strain influencing their stability; smaller rings like cyclopropane have high ring strain causing increased reactivity, whereas larger rings demonstrate enhanced stability and lower reactivity. The difference in spatial arrangement impacts properties such as boiling points, solubility, and acidity, with cyclic structures often showing higher boiling points due to restricted rotation and stronger intermolecular forces.
Occurrence in Nature and Industry
Open-chain compounds are commonly found in natural sources such as carbohydrates, fatty acids, and amino acids, playing a crucial role in biological processes and metabolic pathways. Cyclic compounds, including aromatic rings and cycloalkanes, frequently occur in natural products like steroids, alkaloids, and many vitamins, offering structural stability and unique chemical properties exploited in pharmaceuticals and agrochemicals. Industrially, open-chain molecules are often used in polymer production and fine chemicals, while cyclic compounds are key in synthetic chemistry for creating dyes, resins, and active pharmaceutical ingredients due to their diverse reactivity and complex architectures.
Uses and Applications in Chemistry
Open-chain compounds, characterized by linear or branched structures, are widely used in pharmaceuticals, polymers, and solvents due to their flexibility and ease of functionalization. Cyclic compounds, including aromatic and non-aromatic rings, exhibit enhanced stability and unique reactivity, making them essential in drug design, agrochemicals, and material science. Both structural types play critical roles in organic synthesis, catalysis, and biochemical processes, with cyclic compounds often forming the core of biologically active molecules.
Summary: Choosing Between Open-chain and Cyclic Structures
Open-chain structures offer flexibility and easier modification due to their linear arrangement, making them ideal for applications requiring rapid functionalization and diverse bonding patterns. Cyclic structures provide enhanced stability and rigidity, often resulting in unique chemical and physical properties desirable in pharmaceuticals and materials science. Selecting between open-chain and cyclic compounds depends on the desired molecular stability, reactivity, and the specific functional context of the application.
Open-chain Infographic
 libterm.com
libterm.com