Ionic is a powerful open-source framework for building cross-platform mobile applications using web technologies like HTML, CSS, and JavaScript. It allows developers to create highly performant apps that run seamlessly on iOS, Android, and the web with a single codebase. Explore this article to discover how Ionic can streamline your app development process and enhance user experience.
Table of Comparison
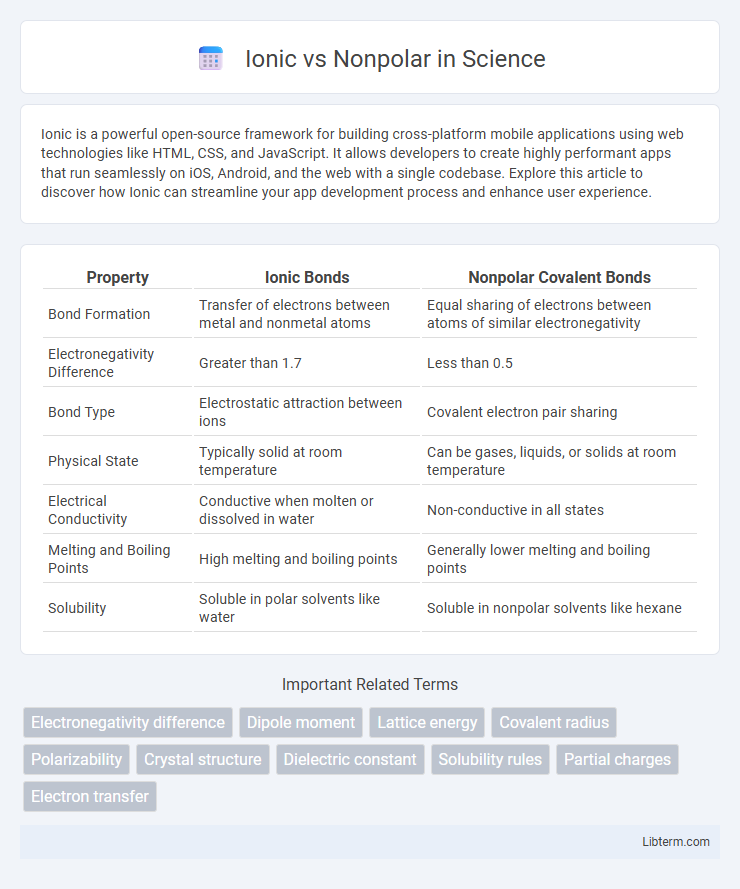
| Property | Ionic Bonds | Nonpolar Covalent Bonds |
|---|---|---|
| Bond Formation | Transfer of electrons between metal and nonmetal atoms | Equal sharing of electrons between atoms of similar electronegativity |
| Electronegativity Difference | Greater than 1.7 | Less than 0.5 |
| Bond Type | Electrostatic attraction between ions | Covalent electron pair sharing |
| Physical State | Typically solid at room temperature | Can be gases, liquids, or solids at room temperature |
| Electrical Conductivity | Conductive when molten or dissolved in water | Non-conductive in all states |
| Melting and Boiling Points | High melting and boiling points | Generally lower melting and boiling points |
| Solubility | Soluble in polar solvents like water | Soluble in nonpolar solvents like hexane |
Introduction to Chemical Bonding
Ionic bonds form through the electrostatic attraction between positively charged cations and negatively charged anions, typically between metals and nonmetals, resulting in the transfer of electrons and the creation of charged ions. Nonpolar covalent bonds occur when two atoms, usually nonmetals with similar electronegativities, share electrons equally, producing a balanced distribution of electron density. Understanding the differences in electron distribution, bond polarity, and resulting molecular properties is crucial in the study of chemical bonding and material behavior.
What Are Ionic Compounds?
Ionic compounds consist of positively charged cations and negatively charged anions held together by strong electrostatic forces, forming a crystalline lattice structure. These compounds typically result from the transfer of electrons between metals and nonmetals, leading to high melting and boiling points due to their ionic bonds. Ionic compounds are usually soluble in water and conduct electricity when molten or dissolved, distinguishing them from nonpolar compounds, which lack such ionic interactions.
Overview of Nonpolar Compounds
Nonpolar compounds consist primarily of molecules with evenly distributed electron density, resulting in minimal or no permanent dipole moment. These compounds are typically formed from nonmetal atoms with similar electronegativities, such as hydrocarbons, and exhibit low solubility in polar solvents like water. Their intermolecular interactions are dominated by London dispersion forces, influencing physical properties such as melting and boiling points.
Key Differences Between Ionic and Nonpolar Bonds
Ionic bonds form through the complete transfer of electrons between atoms, resulting in charged ions that attract each other, whereas nonpolar bonds involve the equal sharing of electrons between atoms, creating neutral molecules. Ionic compounds typically exhibit high melting and boiling points due to strong electrostatic forces, in contrast to nonpolar molecules that have lower melting and boiling points because of weaker Van der Waals interactions. Electrical conductivity also differentiates them; ionic compounds conduct electricity when melted or dissolved in water, while nonpolar substances remain nonconductive in most states.
Properties of Ionic vs Nonpolar Substances
Ionic substances exhibit high melting and boiling points due to strong electrostatic forces between charged ions, making them typically solid and brittle at room temperature. Nonpolar substances have low melting and boiling points because of weak London dispersion forces, resulting in liquids or gases under standard conditions. Ionic compounds conduct electricity when molten or dissolved in water, whereas nonpolar substances are generally poor conductors of electricity.
Examples of Ionic and Nonpolar Compounds
Sodium chloride (NaCl) is a classic example of an ionic compound formed by the transfer of electrons between sodium and chlorine atoms, resulting in strong electrostatic forces. In contrast, nonpolar compounds like methane (CH4) and oxygen (O2) feature covalent bonds with equal sharing of electrons, leading to symmetric electron distribution and minimal polarity. Understanding these examples helps illustrate fundamental differences in bonding, electrical conductivity, solubility, and physical properties between ionic and nonpolar compounds.
Methods to Identify Ionic and Nonpolar Compounds
Ionic compounds can be identified by their high melting points, solubility in water, and electrical conductivity in molten or dissolved states, distinguishing them from nonpolar compounds that typically exhibit low melting and boiling points with poor solubility in polar solvents. Nonpolar compounds often dissolve well in nonpolar solvents like hexane and show no electrical conductivity due to the absence of charged particles. Techniques such as electrical conductivity tests, solubility experiments, and melting point analysis provide reliable methods for differentiating ionic and nonpolar substances.
Real-World Applications and Importance
Ionic compounds, such as sodium chloride, are crucial in applications requiring high solubility in water and electrical conductivity, making them essential in biological systems and industrial electrolytes. Nonpolar substances like oils and fats are vital in pharmaceuticals and cosmetics due to their ability to dissolve nonpolar molecules and form hydrophobic barriers. Understanding the differences between ionic and nonpolar interactions enables the design of materials for targeted drug delivery, water purification, and energy storage solutions.
Advantages and Disadvantages of Each Type
Ionic bonds offer high melting points and excellent electrical conductivity in molten or dissolved states, but they tend to be brittle and soluble only in polar solvents. Nonpolar covalent bonds provide flexibility and lower melting points, making them ideal for organic compounds and materials like oils and fats, though they exhibit poor electrical conductivity and limited solubility in water. Each bonding type suits distinct applications due to these contrasting physical properties and chemical behaviors.
Conclusion: Choosing Between Ionic and Nonpolar Compounds
The choice between ionic and nonpolar compounds depends on the intended application and chemical properties required. Ionic compounds exhibit high melting points and electrical conductivity in molten or dissolved states, making them ideal for use in electrolytes and ceramics. Nonpolar compounds, characterized by low polarity and solubility in nonpolar solvents, are preferred in applications such as organic solvents and nonpolar coatings.
Ionic Infographic
 libterm.com
libterm.com