Neutron emission is a nuclear process where an unstable nucleus releases one or more neutrons to achieve a more stable state. This phenomenon plays a critical role in nuclear reactions, reactor physics, and radioactive decay chains, influencing both energy production and radiation safety. Explore the article to understand how neutron emission impacts nuclear technology and your safety measures.
Table of Comparison
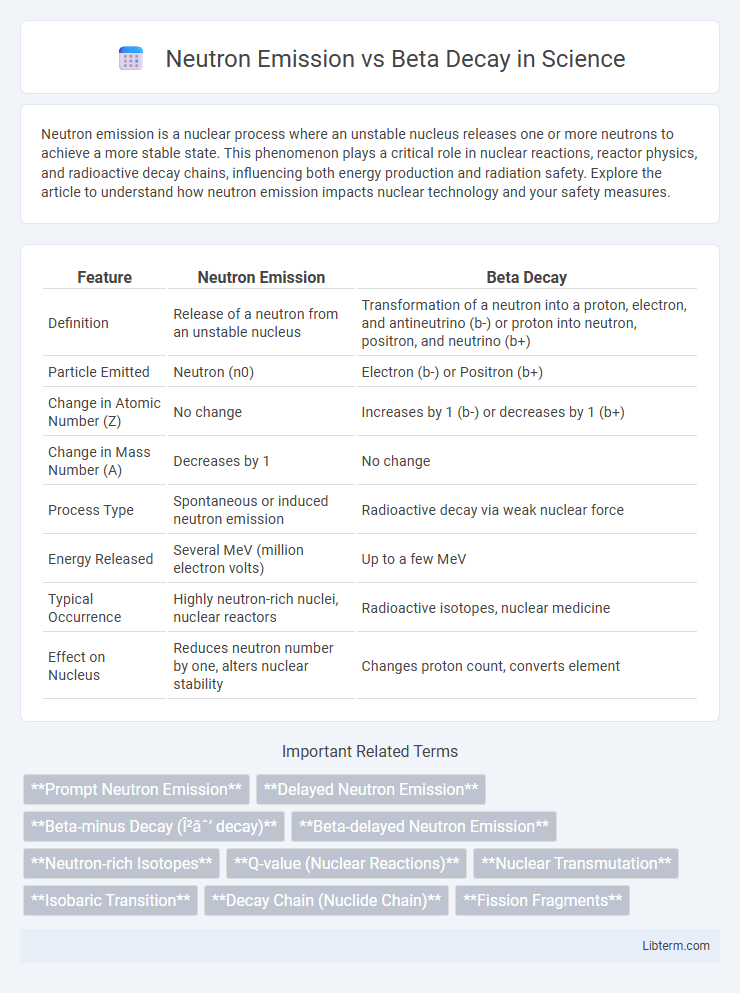
| Feature | Neutron Emission | Beta Decay |
|---|---|---|
| Definition | Release of a neutron from an unstable nucleus | Transformation of a neutron into a proton, electron, and antineutrino (b-) or proton into neutron, positron, and neutrino (b+) |
| Particle Emitted | Neutron (n0) | Electron (b-) or Positron (b+) |
| Change in Atomic Number (Z) | No change | Increases by 1 (b-) or decreases by 1 (b+) |
| Change in Mass Number (A) | Decreases by 1 | No change |
| Process Type | Spontaneous or induced neutron emission | Radioactive decay via weak nuclear force |
| Energy Released | Several MeV (million electron volts) | Up to a few MeV |
| Typical Occurrence | Highly neutron-rich nuclei, nuclear reactors | Radioactive isotopes, nuclear medicine |
| Effect on Nucleus | Reduces neutron number by one, alters nuclear stability | Changes proton count, converts element |
Overview of Nuclear Decay Processes
Nuclear decay processes involve the transformation of unstable atomic nuclei to achieve greater stability, primarily through neutron emission or beta decay. Neutron emission occurs when an excited nucleus releases a neutron, reducing its neutron number without changing its proton count, often seen in neutron-rich isotopes. Beta decay involves the conversion of a neutron into a proton with the emission of an electron (beta-minus) or a proton into a neutron with the emission of a positron (beta-plus), altering the atomic number and resulting in the transmutation of elements.
What is Neutron Emission?
Neutron emission is a type of nuclear decay where an unstable atomic nucleus releases one or more neutrons to achieve a more stable state, typically occurring in neutron-rich isotopes. This process reduces the neutron-to-proton ratio, altering the nuclear composition without changing the atomic number, unlike beta decay which converts a neutron to a proton or vice versa through the emission of electrons or positrons. Neutron emission influences the stability and transmutation of heavy elements, playing a key role in nuclear reactor physics and nucleosynthesis in astrophysical environments.
Understanding Beta Decay
Beta decay is a radioactive process where a neutron in an unstable nucleus transforms into a proton while emitting an electron (beta particle) and an antineutrino, increasing the atomic number by one. This transformation allows the nucleus to achieve greater stability without changing its mass number, differentiating it from neutron emission which reduces the mass number by releasing a free neutron. Understanding beta decay is essential for nuclear chemistry and physics as it explains the formation of new elements and isotopes in radioactive decay chains and stellar nucleosynthesis.
Key Differences Between Neutron Emission and Beta Decay
Neutron emission involves the ejection of a neutron from an unstable nucleus, altering the atomic mass but not the atomic number, while beta decay transforms a neutron into a proton or vice versa, changing the atomic number and emitting an electron or positron. Neutron emission typically occurs in highly neutron-rich isotopes during nuclear fission or radioactive decay, whereas beta decay happens in isotopes seeking to achieve a more stable neutron-to-proton ratio. The energy spectrum and particle types emitted differ significantly, with neutron emission releasing free neutrons and beta decay producing beta particles and neutrinos.
Nuclear Stability and Types of Decay
Neutron emission occurs in neutron-rich nuclei to restore nuclear stability by releasing excess neutrons, typically observed in highly unstable isotopes beyond the neutron drip line. Beta decay involves the transformation of a neutron into a proton or vice versa via weak interaction, balancing the proton-to-neutron ratio to achieve a more stable nucleus. Both decay types contribute to nuclear stability but differ fundamentally: neutron emission directly expels neutrons, while beta decay changes the nuclear composition through particle transformation.
Mechanisms Triggering Neutron Emission
Neutron emission occurs when an unstable nucleus, often neutron-rich, releases excess neutrons to reach a more stable state following nuclear reactions or radioactive decay processes. This mechanism is typically triggered by the excitation of the nucleus beyond the neutron binding energy, causing the neutron to overcome nuclear forces and escape. Unlike beta decay, which transforms a neutron into a proton or vice versa via weak interaction, neutron emission directly ejects a neutron without changing the proton-to-neutron ratio.
Mechanisms Triggering Beta Decay
Beta decay is triggered by the weak nuclear force, which causes a neutron in the nucleus to transform into a proton while emitting an electron (beta particle) and an antineutrino. This process occurs when a nucleus has an excess of neutrons, leading to an unstable neutron-to-proton ratio that drives the conversion to achieve greater nuclear stability. In contrast, neutron emission involves the direct release of a free neutron from an excited nucleus, typically occurring in neutron-rich isotopes without involving the weak interaction.
Energy and Radiation in Neutron Emission vs Beta Decay
Neutron emission releases energy primarily as kinetic energy of emitted neutrons, resulting in high neutron radiation that can penetrate materials and induce secondary reactions. Beta decay emits beta particles (electrons or positrons) and antineutrinos or neutrinos, with energy distributed between the emitted particles and typically lower penetration compared to neutrons. The distinct energy spectra and radiation types influence shielding requirements and detection methods in nuclear physics and radiation safety.
Real-World Applications and Examples
Neutron emission plays a crucial role in nuclear reactors by sustaining chain reactions through neutron release, enabling efficient energy production. Beta decay is essential in medical diagnostics and treatment, with isotopes like Technetium-99m used in imaging and Iodine-131 in cancer therapy. Both processes are fundamental in nuclear physics research, radioactive dating, and safety protocols in nuclear power plants.
Summary: Choosing Between Neutron Emission and Beta Decay
Neutron emission occurs when an unstable nucleus releases excess neutrons to achieve stability, typically in neutron-rich isotopes, whereas beta decay transforms a neutron into a proton or vice versa to balance the neutron-to-proton ratio. The choice between neutron emission and beta decay depends on the nuclear binding energy and the energy thresholds governing particle emission versus beta particle release. Understanding the nuclear structure and decay energy levels allows precise prediction of which decay mode an isotope will favor to reach a more stable state.
Neutron Emission Infographic
 libterm.com
libterm.com