Acid is a substance with a pH less than 7 that can donate protons or accept electron pairs during chemical reactions. It plays a crucial role in various industrial processes, biological functions, and environmental systems. Explore the rest of the article to understand how acids impact your daily life and their diverse applications.
Table of Comparison
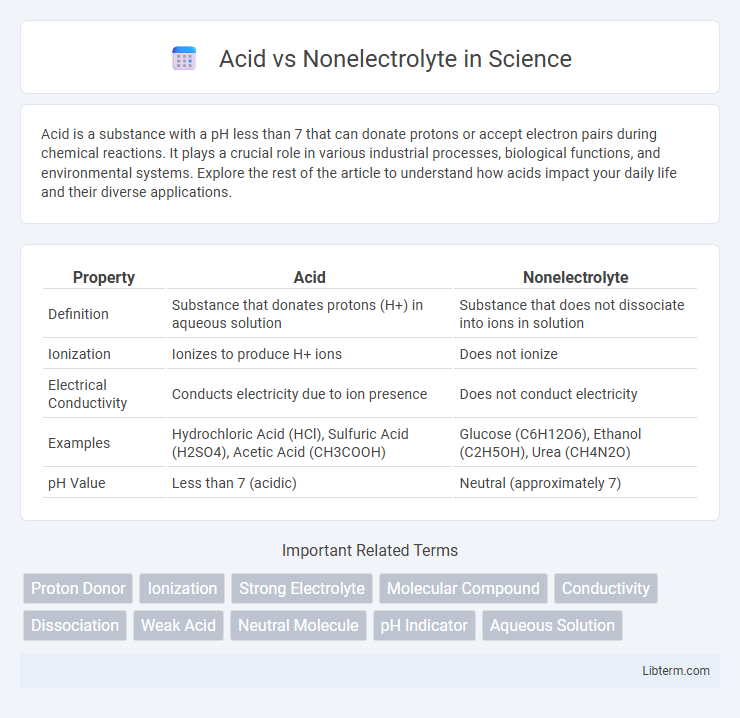
| Property | Acid | Nonelectrolyte |
|---|---|---|
| Definition | Substance that donates protons (H+) in aqueous solution | Substance that does not dissociate into ions in solution |
| Ionization | Ionizes to produce H+ ions | Does not ionize |
| Electrical Conductivity | Conducts electricity due to ion presence | Does not conduct electricity |
| Examples | Hydrochloric Acid (HCl), Sulfuric Acid (H2SO4), Acetic Acid (CH3COOH) | Glucose (C6H12O6), Ethanol (C2H5OH), Urea (CH4N2O) |
| pH Value | Less than 7 (acidic) | Neutral (approximately 7) |
Understanding Acids and Nonelectrolytes
Acids are substances that increase the concentration of hydrogen ions (H+) in aqueous solutions, making them strong electrolytes that conduct electricity efficiently. Nonelectrolytes, such as sugar or ethanol, do not dissociate into ions in water, resulting in no electrical conductivity. Understanding the ionization behavior of acids and the lack thereof in nonelectrolytes is crucial for applications in chemistry, biology, and industrial processes.
Chemical Definitions: Acids vs Nonelectrolytes
Acids are chemical substances that release hydrogen ions (H+) in aqueous solutions, increasing the solution's acidity and enabling it to conduct electricity. Nonelectrolytes, in contrast, do not dissociate into ions in solution, resulting in no electrical conductivity despite being dissolved. The fundamental chemical difference lies in acid ionization versus nonelectrolyte molecular stability without ion formation.
Ionization Behavior in Solution
Acids undergo ionization in solution by donating protons (H+ ions), resulting in the formation of ions that contribute to electrical conductivity. Nonelectrolytes do not ionize or dissociate into ions when dissolved, thus their solutions lack free charged particles and do not conduct electricity. The degree of ionization for acids varies from strong acids, which fully ionize, to weak acids, which partially ionize, whereas nonelectrolytes remain molecular in solution.
Electrical Conductivity Comparison
Acids dissociate in water to release ions, resulting in high electrical conductivity due to the free-moving charged particles. Nonelectrolytes do not ionize in solution, so they exhibit very low or negligible electrical conductivity. The presence of hydrogen ions (H+) in acid solutions is the primary factor enhancing their ability to conduct electricity compared to nonelectrolyte solutions.
Molecular Structure Differences
Acids possess molecular structures that include hydrogen atoms bonded to electronegative elements, enabling ionization in water to release H+ ions, which characterizes their electrolytic behavior. Nonelectrolytes have molecular structures composed of covalent bonds without ionizable hydrogen atoms, preventing dissociation into ions in solution and resulting in no electrical conductivity. The presence or absence of ionizable hydrogen in the molecular framework fundamentally distinguishes acids from nonelectrolytes at the molecular level.
Common Examples of Acids and Nonelectrolytes
Common examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH), which release hydrogen ions (H+) in aqueous solutions. Nonelectrolytes, such as glucose, ethanol, and urea, do not dissociate into ions when dissolved in water, resulting in no electrical conductivity. These distinct chemical properties make acids essential in reactions requiring ionization, while nonelectrolytes serve primarily as molecular solutes without ionic activity.
Physical and Chemical Properties
Acids exhibit distinct physical properties such as a sour taste, ability to turn blue litmus paper red, and typically conduct electricity due to ionization in aqueous solutions, whereas nonelectrolytes do not conduct electricity as they do not dissociate into ions. Chemically, acids release hydrogen ions (H+) in water, engage in reactions with bases forming salts and water, and react with metals producing hydrogen gas, while nonelectrolytes remain chemically neutral and do not participate in ionic reactions. The dissociation constant (Ka) values of acids quantify their strength, a characteristic absent in nonelectrolytes which lack ionizable species in solution.
Real-World Applications and Uses
Acids play a crucial role in industrial processes such as chemical manufacturing, metal cleaning, and battery production due to their ability to donate protons and conduct electricity. Nonelectrolytes, which do not dissociate into ions in solution, are essential in applications like antifreeze fluids, surgical solutions, and food packaging where non-conductive and stable properties are required. Understanding the distinct conductive behaviors of acids and nonelectrolytes enables their targeted use in pharmaceuticals, agriculture, and environmental management.
Safety and Handling Considerations
Handling acids requires stringent safety measures due to their corrosive nature and ability to cause severe burns on contact with skin or eyes, necessitating the use of protective gloves, goggles, and proper ventilation. Nonelectrolytes generally pose less risk in terms of chemical reactivity, but safe handling protocols should still be followed to prevent spills, ingestion, or inhalation hazards depending on the specific compound. Proper storage in labeled containers away from incompatible substances is crucial to prevent dangerous reactions, especially when acids are involved.
Summary Table: Acid vs Nonelectrolyte
Acids ionize in water to produce hydrogen ions (H+), resulting in electrical conductivity, whereas nonelectrolytes do not ionize and thus do not conduct electricity. The summary table highlights that acids exhibit sour taste, react with bases to form salts, and change blue litmus paper to red, while nonelectrolytes lack these properties. Key distinctions include acids' ability to dissociate into ions and participate in acid-base reactions, contrasting with nonelectrolytes' molecular retention and non-conductive nature.
Acid Infographic
 libterm.com
libterm.com