Furanose is a five-membered ring sugar structure commonly found in nucleotides and carbohydrates. Its unique ring formation significantly influences the chemical behavior and biological function of molecules such as RNA and certain polysaccharides. Discover how understanding furanose can enhance your knowledge of biochemical processes by reading the full article.
Table of Comparison
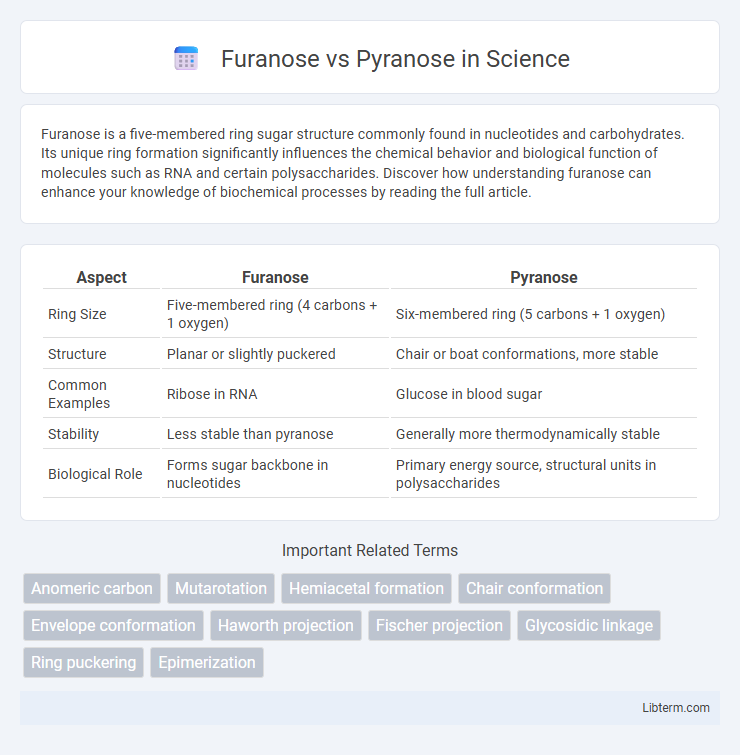
| Aspect | Furanose | Pyranose |
|---|---|---|
| Ring Size | Five-membered ring (4 carbons + 1 oxygen) | Six-membered ring (5 carbons + 1 oxygen) |
| Structure | Planar or slightly puckered | Chair or boat conformations, more stable |
| Common Examples | Ribose in RNA | Glucose in blood sugar |
| Stability | Less stable than pyranose | Generally more thermodynamically stable |
| Biological Role | Forms sugar backbone in nucleotides | Primary energy source, structural units in polysaccharides |
Introduction to Furanose and Pyranose
Furanose and pyranose are cyclic forms of monosaccharides distinguished by the size of their ring structures, five-membered for furanose and six-membered for pyranose. Furanose rings resemble the structure of furan, containing four carbon atoms and one oxygen atom, while pyranose rings resemble pyran, comprising five carbon atoms and one oxygen atom. These ring forms significantly influence the chemical properties, stability, and biological roles of sugars such as ribose and glucose in nucleotides and polysaccharides.
Structural Differences Between Furanose and Pyranose
Furanose and pyranose are two cyclic forms of monosaccharides distinguished by their ring structures; furanose forms a five-membered ring with four carbon atoms and one oxygen atom, while pyranose forms a six-membered ring comprising five carbon atoms and one oxygen atom. This difference in ring size influences the stereochemistry and conformational flexibility of the sugar molecules, affecting their biochemical properties and interactions. The choice of furanose or pyranose form plays a crucial role in carbohydrate chemistry, impacting glycosidic bond formation and enzymatic recognition.
Chemical Formation of Furanose and Pyranose Rings
Furanose rings form through the intramolecular reaction between the carbonyl group on the aldehyde or ketone carbon (typically C1) and the hydroxyl group on the fourth carbon (C4), resulting in a five-membered cyclic hemiacetal or hemiketal structure. Pyranose rings arise from cyclization involving the carbonyl group and the hydroxyl on the fifth carbon (C5), creating a six-membered ring with a hemiacetal or hemiketal linkage. These ring formations stabilize monosaccharides in aqueous solutions, influencing their chemical reactivity and biological recognition.
Common Examples of Furanose and Pyranose Sugars
Furanose sugars commonly include ribose and fructose, which adopt a five-membered ring structure vital in nucleotides and energy metabolism. Pyranose sugars such as glucose and galactose form six-membered rings, playing crucial roles in energy storage and cellular recognition. Both conformations influence the chemical reactivity and biological function of carbohydrates in metabolic pathways.
Stereochemistry and Isomerism in Furanose vs Pyranose
Furanose and pyranose differ significantly in stereochemistry due to their ring sizes, with furanose forming a five-membered ring and pyranose forming a six-membered ring, impacting the spatial arrangement of their substituents. In furanose, the ring adopts envelope conformations, while pyranose typically exhibits chair or boat conformations, influencing stereochemical stability and isomer distribution. Isomerism in furanose and pyranose includes anomeric forms (alpha and beta) arising from the configuration at the anomeric carbon, with pyranoses showing more defined stereochemical variations due to the larger ring and distinct axial-equatorial positions.
Biological Significance of Furanose and Pyranose Forms
Furanose and pyranose forms are crucial in biochemistry, as they represent the ring structures of monosaccharides like ribose and glucose, respectively, affecting their biological functions. Furanose forms, such as ribofuranose in RNA, enable the formation of nucleotides and nucleic acids, essential for genetic information storage and transfer. Pyranose forms, predominantly found in glucose, contribute to energy metabolism and structural components like cellulose and glycogen, highlighting their significance in cellular processes.
Physical Properties and Stability Comparisons
Furanose rings, characterized by five-membered cyclic structures, exhibit greater ring strain compared to six-membered pyranose rings, leading to lower thermodynamic stability. Pyranose forms tend to dominate in solution due to their reduced ring strain and increased conformational flexibility, which enhances overall molecular stability. Physical properties such as melting points and solubility differ, with pyranoses generally displaying higher melting points and lower solubility in water due to their more stable ring conformation.
Analytical Methods for Differentiating Furanose and Pyranose
Nuclear Magnetic Resonance (NMR) spectroscopy provides detailed structural insights by distinguishing the unique chemical shifts and coupling patterns of furanose and pyranose rings. Mass spectrometry, especially when combined with chromatographic separation, aids in identifying ring forms based on fragmentation patterns specific to five-membered (furanose) and six-membered (pyranose) sugars. Infrared (IR) spectroscopy can highlight subtle differences in vibrational modes linked to ring size, supporting differentiation between these carbohydrate forms.
Applications in Biochemistry and Medicine
Furanose and pyranose are cyclic sugar structures critical in biochemistry and medicine, with furanose commonly found in nucleotides such as ribose in RNA, influencing genetic material stability and function. Pyranose forms, prevalent in glucose and other hexoses, play essential roles in energy metabolism and signal transduction pathways. Understanding these ring structures aids in drug design targeting metabolic disorders and contributes to the development of antiviral and anticancer therapies.
Summary: Key Differences and Implications
Furanose structures contain five-membered rings formed by four carbons and one oxygen, while pyranose structures feature six-membered rings composed of five carbons and one oxygen, influencing their conformational stability and chemical reactivity. Furanoses often appear in nucleic acids as ribose or deoxyribose sugars, critical for genetic material integrity, whereas pyranoses are common in glucose and other hexose sugars, vital for energy metabolism. The ring size differences affect glycosidic bond formation and enzyme specificity, impacting biochemical pathways and drug design targeting carbohydrate-binding proteins.
Furanose Infographic
 libterm.com
libterm.com