Hydrolysis is a critical biochemical process where water molecules break down complex compounds into simpler ones, playing a vital role in digestion and cellular functions. This reaction helps convert macromolecules like proteins, carbohydrates, and lipids into absorbable units essential for your body's energy and repair systems. Explore the rest of the article to understand how hydrolysis impacts various biological and industrial applications.
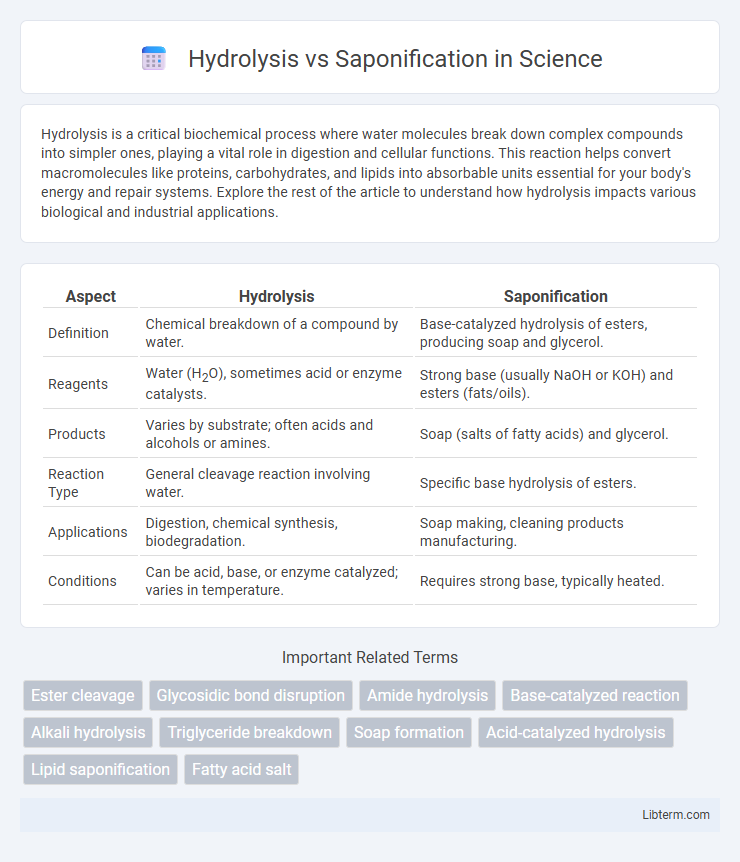
Table of Comparison
| Aspect | Hydrolysis | Saponification |
|---|---|---|
| Definition | Chemical breakdown of a compound by water. | Base-catalyzed hydrolysis of esters, producing soap and glycerol. |
| Reagents | Water (H2O), sometimes acid or enzyme catalysts. | Strong base (usually NaOH or KOH) and esters (fats/oils). |
| Products | Varies by substrate; often acids and alcohols or amines. | Soap (salts of fatty acids) and glycerol. |
| Reaction Type | General cleavage reaction involving water. | Specific base hydrolysis of esters. |
| Applications | Digestion, chemical synthesis, biodegradation. | Soap making, cleaning products manufacturing. |
| Conditions | Can be acid, base, or enzyme catalyzed; varies in temperature. | Requires strong base, typically heated. |
Introduction to Hydrolysis and Saponification
Hydrolysis is a chemical reaction involving the breaking of a bond in a molecule using water, commonly seen in the breakdown of esters into acids and alcohols. Saponification is a specific type of hydrolysis where triglycerides react with a strong base, such as sodium hydroxide, to form glycerol and soap. Both processes are essential in organic chemistry and industrial applications for producing fatty acid derivatives and cleansing agents.
Chemical Definitions: Hydrolysis vs Saponification
Hydrolysis is a chemical reaction where water breaks down compounds into smaller molecules by cleaving bonds, often catalyzed by acids, bases, or enzymes. Saponification is a specific type of hydrolysis involving the reaction of a fat or oil with a strong base, typically sodium hydroxide, resulting in glycerol and soap. While hydrolysis refers to a broad class of water-induced cleavage reactions, saponification specifically describes the base-catalyzed hydrolysis of esters in triglycerides.
Key Differences Between Hydrolysis and Saponification
Hydrolysis is a chemical reaction involving the breakdown of compounds by water, typically catalyzed by acids, bases, or enzymes, whereas saponification is a specific type of base-catalyzed hydrolysis that transforms triglycerides into glycerol and soap molecules. The primary difference lies in the reactants and products: hydrolysis applies broadly to various substrates, while saponification specifically targets fats or oils producing soap. Saponification requires strong alkali agents like sodium hydroxide, whereas hydrolysis may occur under milder acidic or enzymatic conditions.
Mechanisms of Hydrolysis Reactions
Hydrolysis reactions involve the cleavage of chemical bonds through the addition of water molecules, typically breaking esters, amides, or glycosidic linkages. Enzymatic hydrolysis, such as that catalyzed by lipases or proteases, proceeds via nucleophilic attack by water facilitated by the enzyme's active site residues. In contrast, saponification specifically refers to the base-catalyzed hydrolysis of esters, where hydroxide ions attack the carbonyl carbon, yielding an alcohol and a carboxylate salt as products.
Mechanisms of Saponification Reactions
Saponification mechanisms involve the base-catalyzed hydrolysis of esters, where hydroxide ions attack the carbonyl carbon, forming a tetrahedral intermediate that subsequently breaks down into an alcohol and a carboxylate ion. This reaction typically occurs under alkaline conditions, using sodium or potassium hydroxide, resulting in soap formation from triglycerides. Unlike acid-catalyzed hydrolysis, saponification produces a salt of the fatty acid, enhancing soap's solubility in water and its cleansing properties.
Industrial and Laboratory Applications
Hydrolysis and saponification are crucial chemical processes widely used in both industrial and laboratory settings, with hydrolysis primarily breaking down complex molecules such as esters and amides into simpler compounds using water and catalysts. Saponification specifically refers to the hydrolysis of triglycerides with a strong alkali, producing glycerol and soap, making it essential for commercial soap manufacturing and biodiesel production. In laboratories, hydrolysis aids in analyzing molecular structures, while saponification serves as a key step in quantitative fat analysis and synthetic chemistry experiments.
Real-world Examples and Use Cases
Hydrolysis is a chemical process where water breaks down compounds, commonly observed in the digestion of proteins and carbohydrates in the human body. Saponification, a specific type of hydrolysis, involves the reaction of fats or oils with a strong base like sodium hydroxide to produce soap, widely used in detergent and personal care industries. Real-world applications include industrial wastewater treatment utilizing hydrolysis for pollutant breakdown and the soap manufacturing sector relying on saponification to create bar and liquid soaps.
Factors Influencing Each Process
Hydrolysis rate depends heavily on pH, temperature, and enzyme or catalyst presence, where acidic or basic conditions accelerate the breakdown of esters or lipids into their components. Saponification specifically requires a strong base, typically sodium hydroxide or potassium hydroxide, and factors such as reactant concentration, temperature, and mixing efficiency significantly influence soap yield and quality. Both processes are sensitive to reactant purity and the physical state of substrates, with saponification favoring triglycerides in fats and oils while hydrolysis can target a broader range of susceptible compounds.
Environmental Impact and Safety Concerns
Hydrolysis typically involves breaking down compounds using water, often requiring acidic or enzymatic catalysts that pose minimal environmental hazards. Saponification, a specific type of hydrolysis involving alkalis like sodium hydroxide, generates soap and glycerol but can result in caustic waste with potential risks to aquatic life if improperly managed. Both processes demand careful handling to mitigate chemical exposure and ensure compliance with environmental regulations, promoting safer industrial and household applications.
Conclusion: Choosing Between Hydrolysis and Saponification
Hydrolysis breaks down compounds by reacting with water, often requiring acidic or enzymatic catalysts, and is ideal for processes needing mild conditions or specific molecular modifications. Saponification specifically involves the base-catalyzed hydrolysis of esters, producing soap and glycerol, making it the preferred method for soap manufacturing and related industrial applications. Selecting between hydrolysis and saponification depends on the desired product, reaction conditions, and the nature of the starting materials.
Hydrolysis Infographic
 libterm.com
libterm.com