Luminescence is the emission of light by a substance not resulting from heat, often occurring through chemical reactions, electrical energy, or biological processes. It plays a crucial role in various applications such as medical imaging, display technologies, and environmental monitoring. Explore the article to discover how luminescence impacts your daily life and its exciting future developments.
Table of Comparison
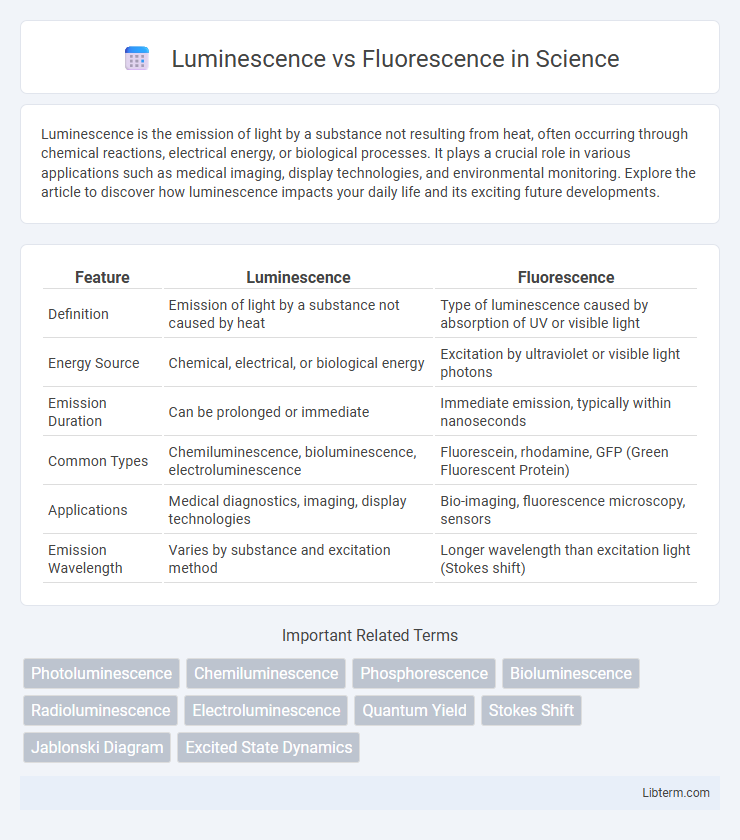
| Feature | Luminescence | Fluorescence |
|---|---|---|
| Definition | Emission of light by a substance not caused by heat | Type of luminescence caused by absorption of UV or visible light |
| Energy Source | Chemical, electrical, or biological energy | Excitation by ultraviolet or visible light photons |
| Emission Duration | Can be prolonged or immediate | Immediate emission, typically within nanoseconds |
| Common Types | Chemiluminescence, bioluminescence, electroluminescence | Fluorescein, rhodamine, GFP (Green Fluorescent Protein) |
| Applications | Medical diagnostics, imaging, display technologies | Bio-imaging, fluorescence microscopy, sensors |
| Emission Wavelength | Varies by substance and excitation method | Longer wavelength than excitation light (Stokes shift) |
Introduction to Luminescence and Fluorescence
Luminescence is the emission of light by a substance not resulting from heat, involving various mechanisms such as chemiluminescence, bioluminescence, and photoluminescence. Fluorescence, a type of photoluminescence, occurs when a material absorbs photons and rapidly re-emits them as visible light, typically within nanoseconds. These processes are critical in applications like bioimaging, chemical sensing, and display technologies due to their distinct emission properties and excitation requirements.
Defining Luminescence: Key Concepts
Luminescence refers to the emission of light by a substance that has absorbed energy, occurring without significant heat production, and can arise from various processes including chemical reactions, electrical energy, or subatomic motions. It encompasses multiple types such as chemiluminescence, bioluminescence, and photoluminescence, where the latter involves light absorption and subsequent light emission. Key concepts defining luminescence include energy absorption, excited electronic states, and the release of photons as the material returns to its ground state.
Understanding Fluorescence: Essential Principles
Fluorescence occurs when a substance absorbs high-energy photons, typically in the ultraviolet or visible spectrum, and then emits light at a longer wavelength almost instantaneously, within nanoseconds. This photoluminescent process involves the rapid transition of electrons from an excited singlet state back to the ground state, releasing energy as visible light. Key principles include the Stokes shift, which is the difference between absorption and emission wavelengths, and quantum yield, measuring fluorescence efficiency critical for applications in bioimaging and chemical sensing.
Types of Luminescence: Beyond Fluorescence
Types of luminescence extend beyond fluorescence to include phosphorescence, chemiluminescence, and electroluminescence, each characterized by distinct emission mechanisms and time scales. Phosphorescence involves long-lived excited states causing delayed light emission, unlike the rapid photon release seen in fluorescence. Chemiluminescence arises from chemical reactions producing light without external illumination, while electroluminescence occurs when electric current energizes materials to emit photons, demonstrating the diverse origins of luminescent phenomena.
Main Differences Between Luminescence and Fluorescence
Luminescence is a broader phenomenon where a substance emits light due to any form of excitation, including chemical reactions, electrical energy, or radiation, while fluorescence is a specific type of luminescence caused by the absorption of ultraviolet or visible light followed by the rapid emission of light at a longer wavelength. Unlike luminescence, fluorescence occurs only during exposure to an excitation source and stops immediately when the source is removed, with emission lifetimes typically in the nanosecond range. Luminescence includes various subtypes such as chemiluminescence, bioluminescence, and phosphorescence, whereas fluorescence is characterized by its prompt and short-lived emitted light.
Mechanisms of Luminescence: How It Works
Luminescence occurs when a material absorbs energy and re-emits it as light through electronic transitions in its atoms or molecules, bypassing heat generation. The process involves excitation of electrons to higher energy states followed by radiative decay, releasing photons at characteristic wavelengths. This mechanism contrasts with fluorescence, where emission happens rapidly after excitation and ceases almost immediately once the energy source is removed.
Fluorescence Mechanism: Step-by-Step Process
Fluorescence occurs when a molecule absorbs photons, causing its electrons to transition from the ground state to an excited singlet state. These excited electrons rapidly lose some energy through vibrational relaxation and internal conversion before returning to the ground state, emitting photons with longer wavelengths than the absorbed light. This step-by-step process involves excitation, non-radiative relaxation, and radiative emission, distinguishing fluorescence from other luminescence types by its short-lived emission typically lasting nanoseconds.
Applications of Luminescence in Science and Industry
Luminescence encompasses a range of light-emitting phenomena, including fluorescence, and finds extensive applications in science and industry such as biochemical assays, environmental monitoring, and medical diagnostics. Its ability to emit light without heat generation enables highly sensitive detection methods in forensic analysis, DNA sequencing, and pharmaceutical quality control. Advances in luminescent materials also drive innovation in display technology, optical sensors, and energy-efficient lighting systems.
Real-World Uses of Fluorescence Technology
Fluorescence technology is widely used in medical diagnostics, enabling techniques such as fluorescence microscopy for detailed cellular imaging and fluorescence-based assays for detecting biomarkers. It plays a crucial role in environmental monitoring, allowing the detection of pollutants and toxins through fluorescent sensors. In consumer products, fluorescence enhances security features in banknotes and textiles, demonstrating its broad applicability across various industries.
Summary: Comparing Luminescence and Fluorescence
Luminescence encompasses all processes where substances emit light without heating, including fluorescence, phosphorescence, and chemiluminescence, while fluorescence specifically refers to the rapid re-emission of light by a material after absorbing photons. Fluorescence occurs within nanoseconds and stops almost immediately when the excitation source is removed, whereas other luminescent processes may persist longer. Understanding the distinctions between these phenomena is critical in fields such as spectroscopy, bioimaging, and material science for selecting appropriate detection or analysis methods.
Luminescence Infographic
 libterm.com
libterm.com