Mitophagy is a crucial cellular process that selectively removes damaged or dysfunctional mitochondria to maintain cell health and energy balance. This mechanism supports metabolic efficiency and prevents the accumulation of defective mitochondria that can lead to neurodegenerative diseases and other health issues. Discover how understanding mitophagy can enhance your knowledge of cellular health in the rest of the article.
Table of Comparison
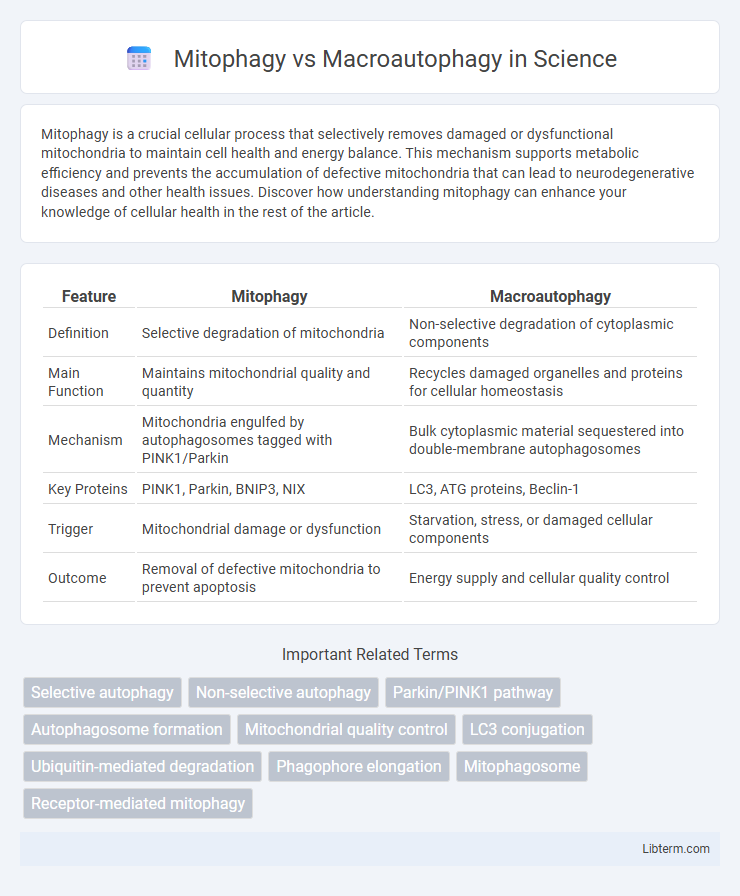
| Feature | Mitophagy | Macroautophagy |
|---|---|---|
| Definition | Selective degradation of mitochondria | Non-selective degradation of cytoplasmic components |
| Main Function | Maintains mitochondrial quality and quantity | Recycles damaged organelles and proteins for cellular homeostasis |
| Mechanism | Mitochondria engulfed by autophagosomes tagged with PINK1/Parkin | Bulk cytoplasmic material sequestered into double-membrane autophagosomes |
| Key Proteins | PINK1, Parkin, BNIP3, NIX | LC3, ATG proteins, Beclin-1 |
| Trigger | Mitochondrial damage or dysfunction | Starvation, stress, or damaged cellular components |
| Outcome | Removal of defective mitochondria to prevent apoptosis | Energy supply and cellular quality control |
Introduction to Autophagy: Key Concepts
Autophagy is a cellular degradation process crucial for maintaining homeostasis by recycling cytoplasmic components. Mitophagy specifically targets damaged or superfluous mitochondria to prevent oxidative stress and maintain mitochondrial quality control, while macroautophagy engulfs bulk cytoplasmic material within double-membrane autophagosomes for lysosomal degradation. Both pathways are regulated by conserved ATG (autophagy-related) proteins and are essential for cellular health and adaptation to metabolic stress.
What is Mitophagy? An Overview
Mitophagy is a selective form of autophagy that specifically targets damaged or dysfunctional mitochondria for degradation, ensuring cellular homeostasis and energy efficiency. This process involves the recognition and sequestration of impaired mitochondria into autophagosomes, which subsequently fuse with lysosomes for degradation. Mitophagy plays a critical role in preventing mitochondrial dysfunction-related diseases and maintaining metabolic balance within cells.
Understanding Macroautophagy
Macroautophagy is a cellular degradation process that engulfs bulk cytoplasmic components, including damaged organelles, into double-membraned autophagosomes for lysosomal degradation. It plays a critical role in maintaining cellular homeostasis, responding to nutrient deprivation, and recycling cellular constituents for energy production. Unlike mitophagy, which selectively targets damaged mitochondria, macroautophagy operates as a broader, non-selective system essential for overall cellular quality control.
Distinct Molecular Pathways: Mitophagy vs Macroautophagy
Mitophagy specifically targets damaged or superfluous mitochondria through PINK1/Parkin-mediated ubiquitination and recruitment of autophagy receptors such as NDP52 and OPTN, facilitating selective mitochondrial degradation. In contrast, macroautophagy involves the formation of double-membrane autophagosomes that nonspecifically sequester cytoplasmic components, regulated by core autophagy proteins like ULK1, Beclin-1, and ATG complexes. These distinct molecular pathways reflect the selective nature of mitophagy for mitochondrial quality control versus the broader cytoplasmic turnover mediated by macroautophagy.
Functional Roles in Cellular Homeostasis
Mitophagy selectively targets damaged or superfluous mitochondria to maintain mitochondrial quality and prevent cellular stress by regulating energy production and apoptosis. Macroautophagy involves the bulk degradation of cytoplasmic components, including organelles and protein aggregates, to recycle nutrients and maintain cellular metabolism during stress or starvation. Both pathways are crucial for cellular homeostasis by balancing organelle turnover, preventing the accumulation of dysfunctional components, and supporting cell survival under varying conditions.
Triggers and Regulatory Mechanisms
Mitophagy is specifically triggered by mitochondrial damage, loss of membrane potential, and oxidative stress, with PINK1 and Parkin proteins acting as key regulators by marking impaired mitochondria for degradation. Macroautophagy is activated by nutrient deprivation and cellular stress, involving ULK1 kinase activation and mTORC1 inhibition to initiate autophagosome formation. Both processes share common regulatory pathways like AMPK activation but differ in selective targeting, as mitophagy specifically removes dysfunctional mitochondria while macroautophagy degrades bulk cytoplasmic components.
Key Proteins and Markers Involved
Mitophagy primarily involves proteins such as PINK1 and Parkin that regulate the selective degradation of damaged mitochondria, while macroautophagy relies on core autophagy proteins including LC3 (microtubule-associated protein 1A/1B-light chain 3) and Beclin-1 to form autophagosomes. Mitophagy-specific receptors like NIX and BNIP3 facilitate mitochondrial recognition, contrasting with the broader cargo receptors like p62/SQSTM1 involved in macroautophagy. Both processes utilize ubiquitination signaling, but the specificity of substrates and adaptors distinguishes mitophagy's targeted clearance from the generalized degradation in macroautophagy.
Physiological and Pathological Implications
Mitophagy selectively degrades damaged mitochondria, maintaining cellular energy homeostasis and preventing oxidative stress, which is crucial for neuroprotection and cardiomyocyte function. In contrast, macroautophagy nonspecifically recycles cytoplasmic components to support nutrient supply during starvation and remove aggregated proteins, with dysfunction linked to cancer and neurodegenerative diseases. Dysregulation of mitophagy or macroautophagy contributes to pathological conditions such as Parkinson's disease, cancer progression, and metabolic disorders by impairing cellular quality control mechanisms.
Therapeutic Potential and Future Directions
Mitophagy selectively eliminates damaged mitochondria, maintaining cellular energy homeostasis and offering targeted therapeutic potential against neurodegenerative diseases and metabolic disorders. Macroautophagy globally degrades cytoplasmic components, including organelles, supporting cell survival under stress and showing promise in treatments for cancer and infectious diseases. Future research aims to refine selective modulation of mitophagy and macroautophagy pathways to enhance specificity and efficacy in personalized medicine.
Conclusion: Comparing Mitophagy and Macroautophagy
Mitophagy specifically targets damaged or superfluous mitochondria for degradation, maintaining mitochondrial quality and cellular energy homeostasis. Macroautophagy involves the sequestration of diverse cytoplasmic components, including organelles and protein aggregates, within autophagosomes for lysosomal degradation. Comparing mitophagy and macroautophagy reveals that mitophagy is a selective form of macroautophagy with a critical role in mitophagic signaling pathways, cellular metabolism, and mitochondrial turnover, highlighting its importance in preventing diseases linked to mitochondrial dysfunction.
Mitophagy Infographic
 libterm.com
libterm.com