Catalysis accelerates chemical reactions by lowering the activation energy, enabling processes to occur more efficiently and with less energy consumption. Inhibition, on the other hand, slows down or halts reactions by interfering with enzyme activity or substrate availability, playing a crucial role in regulating biochemical pathways. Explore the rest of the article to understand how catalysis and inhibition impact your chemical and biological systems.
Table of Comparison
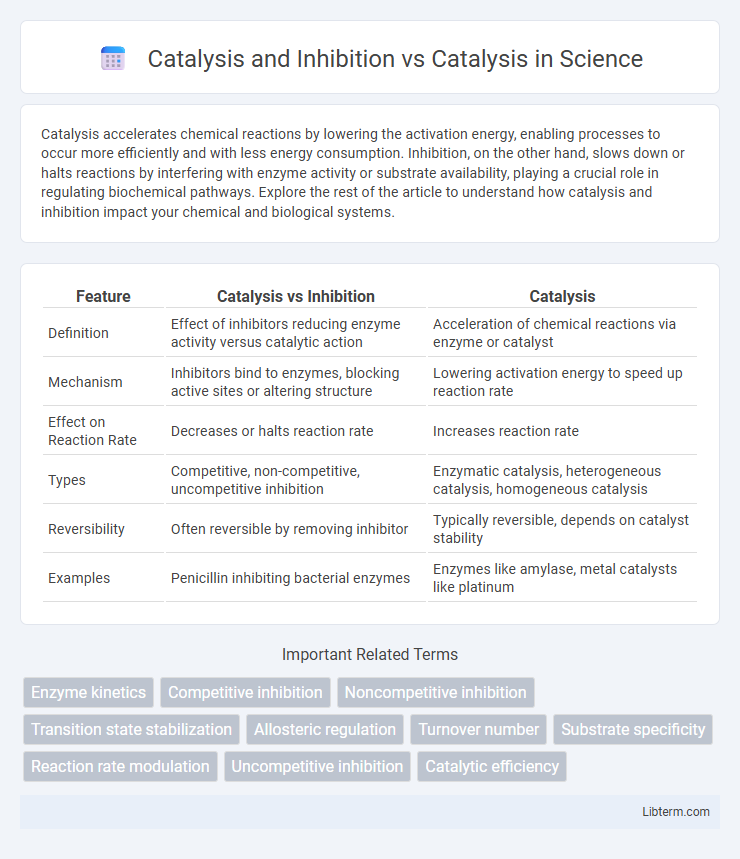
| Feature | Catalysis vs Inhibition | Catalysis |
|---|---|---|
| Definition | Effect of inhibitors reducing enzyme activity versus catalytic action | Acceleration of chemical reactions via enzyme or catalyst |
| Mechanism | Inhibitors bind to enzymes, blocking active sites or altering structure | Lowering activation energy to speed up reaction rate |
| Effect on Reaction Rate | Decreases or halts reaction rate | Increases reaction rate |
| Types | Competitive, non-competitive, uncompetitive inhibition | Enzymatic catalysis, heterogeneous catalysis, homogeneous catalysis |
| Reversibility | Often reversible by removing inhibitor | Typically reversible, depends on catalyst stability |
| Examples | Penicillin inhibiting bacterial enzymes | Enzymes like amylase, metal catalysts like platinum |
Introduction to Catalysis
Catalysis involves accelerating chemical reactions by lowering activation energy through the presence of a catalyst, without the catalyst being consumed. Inhibition contrasts by decreasing reaction rates through molecules that interfere with catalysis or active sites. Understanding catalysis includes studying both positive effects of catalysts and negative effects of inhibitors on reaction mechanisms.
Defining Inhibition in Chemical Reactions
In chemical reactions, inhibition refers to the process that decreases the rate of a catalyzed reaction by interfering with the catalyst's activity. Unlike catalysis, which accelerates reactions by lowering activation energy, inhibition involves molecules called inhibitors that bind to the catalyst or reactant, preventing proper function. Understanding inhibition mechanisms is crucial for controlling reaction rates in industrial processes and biochemical pathways.
Types of Catalysts and Their Functions
Catalysts are substances that increase the rate of chemical reactions without being consumed, classified mainly into homogeneous, heterogeneous, and enzymatic types. Homogeneous catalysts function in the same phase as reactants, facilitating molecular interactions, while heterogeneous catalysts provide surface sites for reactant adsorption and transformation. Inhibition occurs when specific molecules decrease catalytic activity by binding to active sites, altering catalyst structure, or competing with reactants, thereby regulating reaction rates in industrial and biological systems.
Mechanisms of Catalytic Action
Catalysis enhances reaction rates by stabilizing transition states and lowering activation energy through mechanisms such as acid-base catalysis, covalent catalysis, and metal ion catalysis. Inhibition interferes with catalytic mechanisms by binding to active sites or altering enzyme conformation, reducing substrate access or catalytic efficiency. Understanding the distinctions in molecular interactions between catalysis and inhibition is crucial for designing effective enzyme modulators and drugs.
Catalysis vs. Enzyme Inhibition
Catalysis accelerates biochemical reactions by lowering activation energy through enzyme active sites, enhancing substrate conversion to products. Enzyme inhibition restricts or halts catalytic activity by binding inhibitors to the enzyme, either reversibly or irreversibly, impacting metabolic pathways. Understanding the balance between catalysis and enzyme inhibition is crucial for drug design and regulating enzymatic functions in physiological and pathological processes.
Competitive and Non-Competitive Inhibition
Competitive inhibition occurs when an inhibitor molecule resembles the substrate and binds to the enzyme's active site, preventing substrate binding and reducing the reaction rate. Non-competitive inhibition involves an inhibitor binding to an allosteric site, causing a conformational change in the enzyme that decreases its catalytic activity without affecting substrate binding affinity. Both mechanisms are crucial for regulating enzyme activity in metabolic pathways and drug design.
Industrial Applications of Catalysis and Inhibition
Catalysis enhances reaction rates in industrial processes such as petroleum refining and chemical manufacturing by lowering activation energy and increasing efficiency. Inhibition plays a crucial role in controlling undesired side reactions and preventing catalyst deactivation, thereby extending catalyst lifetime and maintaining process stability. Industrial applications leverage both catalysis and inhibition to optimize production yields, reduce energy consumption, and improve product selectivity in sectors like pharmaceuticals, polymers, and environmental technologies.
Catalytic Efficiency and Inhibitor Impact
Catalytic efficiency measures how effectively an enzyme converts substrates to products, typically quantified by the kcat/Km ratio, highlighting the enzyme's speed and affinity. Inhibition negatively affects catalytic efficiency by decreasing the enzyme's ability to bind substrates or catalyze reactions, often through competitive, non-competitive, or uncompetitive mechanisms. Understanding the balance between catalysis and inhibition is crucial for optimizing enzyme functionality in industrial applications and drug design.
Recent Advances in Catalyst and Inhibitor Design
Recent advances in catalyst and inhibitor design have leveraged computational modeling and high-throughput screening to enhance specificity and efficiency in chemical reactions. Innovations in enzyme mimetics and transition metal catalysts have significantly improved catalytic turnover rates, while novel inhibitor molecules are tailored to tightly bind active sites, reducing off-target effects. These developments are driving progress in pharmaceutical synthesis and industrial biocatalysis by enabling precise control over reaction pathways and inhibition mechanisms.
Future Perspectives in Catalysis and Inhibition Research
Future perspectives in catalysis and inhibition research emphasize designing highly selective catalysts that balance activity with specificity to minimize undesired reactions. Advances in computational modeling and machine learning enable precise prediction and tuning of catalyst behavior, accelerating discovery and optimization processes. Emerging applications in sustainable chemistry and pharmaceutical synthesis highlight the critical role of innovative catalysis and inhibition strategies for enhanced efficiency and environmental compatibility.
Catalysis and Inhibition Infographic
 libterm.com
libterm.com