Alpha decay is a type of radioactive decay where an unstable atomic nucleus emits an alpha particle, consisting of two protons and two neutrons. This process reduces the original atom's atomic number by two and mass number by four, leading to the formation of a new element. Discover how alpha decay influences nuclear stability and its applications in medicine and industry by reading the rest of this article.
Table of Comparison
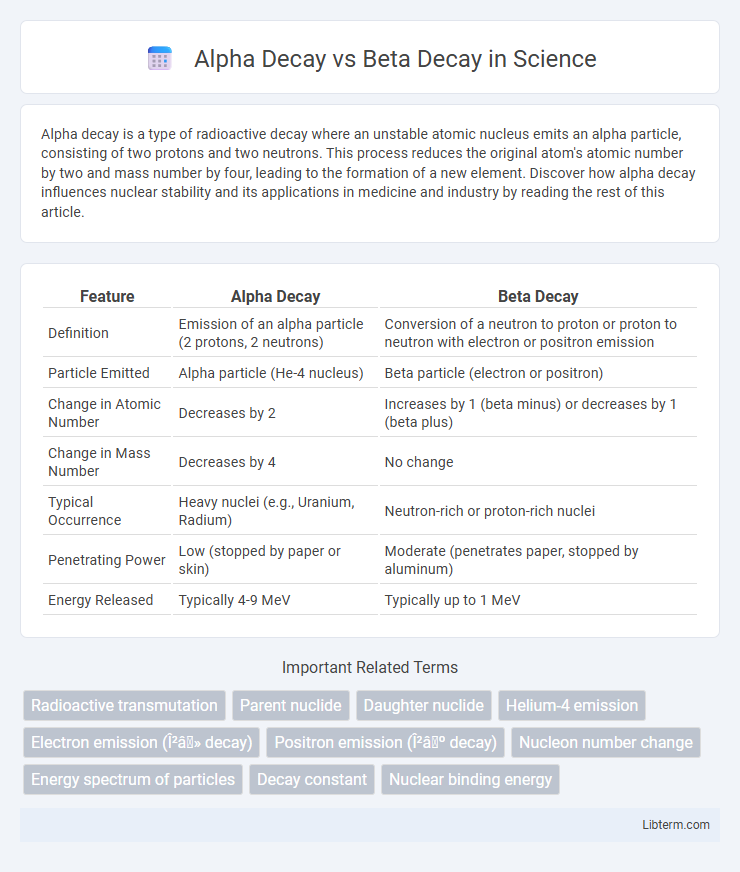
| Feature | Alpha Decay | Beta Decay |
|---|---|---|
| Definition | Emission of an alpha particle (2 protons, 2 neutrons) | Conversion of a neutron to proton or proton to neutron with electron or positron emission |
| Particle Emitted | Alpha particle (He-4 nucleus) | Beta particle (electron or positron) |
| Change in Atomic Number | Decreases by 2 | Increases by 1 (beta minus) or decreases by 1 (beta plus) |
| Change in Mass Number | Decreases by 4 | No change |
| Typical Occurrence | Heavy nuclei (e.g., Uranium, Radium) | Neutron-rich or proton-rich nuclei |
| Penetrating Power | Low (stopped by paper or skin) | Moderate (penetrates paper, stopped by aluminum) |
| Energy Released | Typically 4-9 MeV | Typically up to 1 MeV |
Introduction to Radioactive Decay
Radioactive decay involves the transformation of unstable atomic nuclei into more stable configurations through the emission of particles or radiation. Alpha decay emits an alpha particle consisting of two protons and two neutrons, resulting in a decrease of atomic number by two and mass number by four. Beta decay involves the conversion of a neutron into a proton with the emission of a beta particle (electron or positron), changing the atomic number by one while the mass number remains unchanged.
What is Alpha Decay?
Alpha decay is a radioactive process in which an unstable nucleus emits an alpha particle, consisting of two protons and two neutrons, resulting in the transformation of the original atom into a new element with a mass number reduced by four and atomic number decreased by two. This type of decay commonly occurs in heavy elements such as uranium-238 and radium-226, leading to a decrease in nuclear mass and a change in chemical properties. The emitted alpha particles have low penetration power but high ionizing capability, making alpha decay significant in nuclear physics and radiometric dating.
What is Beta Decay?
Beta decay is a type of radioactive decay where a neutron in an unstable nucleus transforms into a proton, emitting a beta particle, which can be an electron (beta-minus decay) or a positron (beta-plus decay). This process increases or decreases the atomic number of the nucleus by one, altering the element while conserving the mass number. Beta decay plays a crucial role in nuclear transmutation and is characterized by the weak nuclear force mediating the transformation.
Mechanisms of Alpha Decay
Alpha decay involves the emission of an alpha particle, consisting of two protons and two neutrons, from an unstable atomic nucleus, resulting in the formation of a new nucleus with a mass number reduced by four and atomic number decreased by two. This quantum tunneling process enables the alpha particle to overcome the nuclear potential barrier despite lacking the classical energy to escape. In contrast, beta decay involves the transformation of a neutron into a proton or vice versa with the emission of beta particles (electrons or positrons), altering the atomic number without significantly changing the mass number.
Mechanisms of Beta Decay
Beta decay involves the transformation of a neutron into a proton or a proton into a neutron within an atomic nucleus, accompanied by the emission of a beta particle (electron or positron) and an antineutrino or neutrino. This process changes the atomic number by one unit, altering the element while conserving the mass number. Unlike alpha decay, which emits a helium nucleus, beta decay occurs through the weak nuclear force and results in the conversion of quarks inside nucleons, enabling the transmutation of elements in radioactive isotopes.
Key Differences: Alpha vs Beta Decay
Alpha decay involves the emission of an alpha particle consisting of two protons and two neutrons, resulting in a decrease in both atomic number and mass number of the parent nucleus. Beta decay entails the transformation of a neutron into a proton or vice versa, accompanied by the emission of a beta particle (electron or positron) and a neutrino, altering the atomic number by one while keeping the mass number constant. Alpha decay typically occurs in heavy elements like uranium and thorium, whereas beta decay is common in neutron-rich or proton-rich isotopes seeking a more stable nuclear configuration.
Alpha and Beta Decay: Effects on Atomic Nucleus
Alpha decay reduces the atomic number by 2 and the mass number by 4, emitting a helium nucleus and resulting in a new element with a significantly altered nucleus. Beta decay transforms a neutron into a proton or vice versa within the nucleus, changing the atomic number by +-1 while leaving the mass number nearly unchanged. These processes alter nuclear stability and transmute elements, profoundly impacting atomic structure.
Common Isotopes Undergoing Alpha and Beta Decay
Common isotopes undergoing alpha decay include Uranium-238, Thorium-232, and Radium-226, which release helium nuclei to transform into lighter elements. Beta decay frequently occurs in isotopes like Carbon-14, Tritium (Hydrogen-3), and Iodine-131, involving the conversion of a neutron into a proton or vice versa, accompanied by the emission of electrons or positrons. These decay processes play critical roles in nuclear physics, radiometric dating, and medical applications.
Applications in Medicine and Industry
Alpha decay is utilized in targeted cancer therapies, specifically in alpha-particle radiotherapy, where alpha emitters like radium-223 selectively destroy cancer cells with minimal damage to surrounding tissue. Beta decay plays a critical role in medical diagnostics and treatment, with beta-emitting isotopes such as iodine-131 widely used in thyroid cancer treatment and technetium-99m employed for imaging. Industrial applications leverage alpha decay for static eliminators and smoke detectors, while beta decay facilitates thickness gauging and radiotracers in leak detection.
Summary: Comparing Alpha and Beta Decay
Alpha decay emits an alpha particle consisting of two protons and two neutrons, reducing the atomic number by two and mass number by four, whereas beta decay involves the transformation of a neutron into a proton or vice versa, emitting a beta particle (electron or positron) that changes the atomic number by one without altering the mass number. Alpha decay primarily occurs in heavy nuclei, leading to significant changes in nuclear composition, while beta decay occurs in neutron-rich or proton-rich nuclei, adjusting the proton-to-neutron ratio for nuclear stability. Both decay types contribute to radioactive decay chains, but differ in particle emission, energy release, and impact on the nucleus structure.
Alpha Decay Infographic
 libterm.com
libterm.com