Hydrophobic materials repel water, preventing moisture from adhering to their surfaces and enhancing durability in wet environments. These properties are crucial in applications such as outdoor gear, electronics, and building materials to protect against water damage. Discover how hydrophobic technology can improve your everyday products in the rest of this article.
Table of Comparison
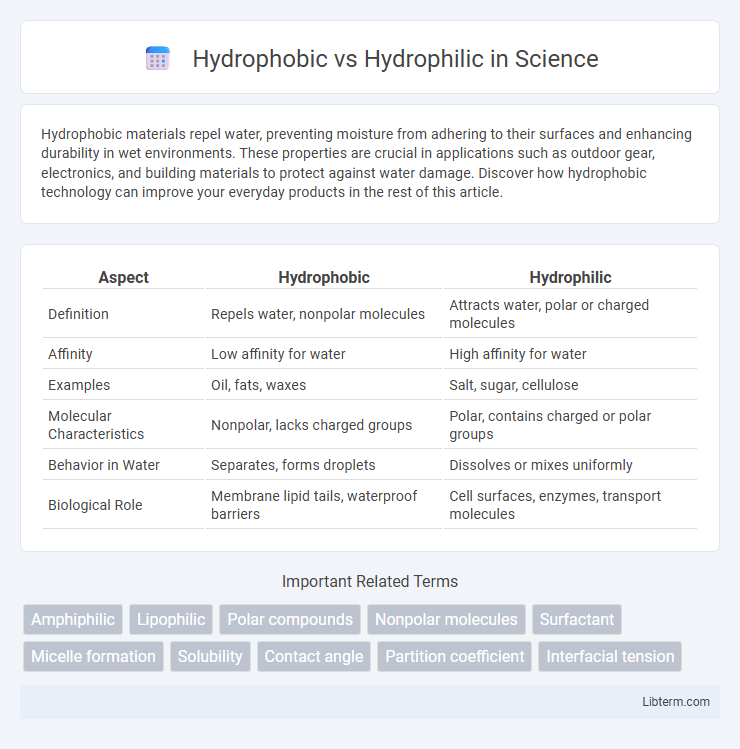
| Aspect | Hydrophobic | Hydrophilic |
|---|---|---|
| Definition | Repels water, nonpolar molecules | Attracts water, polar or charged molecules |
| Affinity | Low affinity for water | High affinity for water |
| Examples | Oil, fats, waxes | Salt, sugar, cellulose |
| Molecular Characteristics | Nonpolar, lacks charged groups | Polar, contains charged or polar groups |
| Behavior in Water | Separates, forms droplets | Dissolves or mixes uniformly |
| Biological Role | Membrane lipid tails, waterproof barriers | Cell surfaces, enzymes, transport molecules |
Introduction to Hydrophobic and Hydrophilic Properties
Hydrophobic substances repel water due to nonpolar molecular structures that do not form hydrogen bonds with water molecules, leading to water avoidance and phase separation. Hydrophilic substances attract and interact with water through polar or charged groups, enabling solubility and surface adhesion in aqueous environments. Understanding these properties is essential for applications in material science, biology, and chemistry, influencing phenomena such as membrane formation and drug delivery.
Defining Hydrophobic and Hydrophilic Substances
Hydrophobic substances repel water due to their nonpolar molecular structure, causing them to avoid interactions with water molecules, as seen in oils and waxes. Hydrophilic substances attract and interact readily with water through polar or charged groups, enabling solubility, commonly observed in substances like salts and sugars. The differing affinities influence molecular behavior in biological membranes, emulsions, and surface chemistry applications.
Molecular Structure and Behavior in Water
Hydrophobic molecules typically consist of nonpolar covalent bonds, resulting in a lack of affinity for water molecules and causing them to aggregate away from aqueous environments. Hydrophilic molecules contain polar or charged functional groups such as hydroxyl, carboxyl, or amino groups, which form hydrogen bonds or ionic interactions with water, enhancing their solubility. This difference in molecular structure determines their distinct behaviors in water, where hydrophilic substances dissolve readily while hydrophobic compounds phase-separate or cluster to minimize water contact.
Key Differences Between Hydrophobic and Hydrophilic Materials
Hydrophobic materials repel water due to nonpolar molecular structures, leading to low surface energy and poor wettability, whereas hydrophilic materials attract water because of polar or charged groups that enhance adhesion and absorption. Hydrophobic substances, such as oils and waxes, resist water interaction and often form droplets on surfaces, contrasting with hydrophilic substances like cellulose or salt that readily dissolve or absorb water. These differences significantly impact applications in coatings, textiles, and biomaterials where water resistance or water affinity is critical.
Common Examples in Nature and Industry
Hydrophobic substances repel water and are commonly found in natural examples like wax coatings on leaves and animal fur oils, which provide water resistance. Hydrophilic materials attract and absorb water, illustrated by cellulose in plant cell walls and industrial uses such as superabsorbent polymers in hygiene products. These opposing properties are crucial in applications ranging from waterproof clothing to water purification technologies.
Applications in Science and Technology
Hydrophobic materials repel water and are essential in applications like waterproof coatings, self-cleaning surfaces, and corrosion resistance in electronics. Hydrophilic substances attract water, enhancing their use in biomedical devices, drug delivery systems, and water purification membranes. Advanced research in nanotechnology leverages hydrophobic and hydrophilic properties to develop responsive materials for sensors and smart textiles.
Impact on Biological Systems and Processes
Hydrophobic molecules repel water, influencing cell membrane integrity by forming lipid bilayers that compartmentalize cellular functions and regulate substance transport. Hydrophilic molecules attract water, facilitating essential processes such as nutrient absorption, enzymatic reactions, and intracellular signaling in aqueous environments. The balance between hydrophobic and hydrophilic interactions is critical for protein folding, membrane permeability, and maintaining cellular homeostasis in biological systems.
Role in Surface Coatings and Textile Engineering
Hydrophobic surfaces repel water, enhancing stain resistance and durability in surface coatings and textiles, making them ideal for outdoor gear and waterproof clothing. Hydrophilic coatings promote moisture absorption and breathability, improving comfort and dyeability in fabrics used for sportswear and medical textiles. Advances in nanotechnology allow precise control over hydrophobic and hydrophilic properties, optimizing performance tailored to specific textile engineering applications.
Testing and Measuring Surface Properties
Surface properties like hydrophobicity and hydrophilicity are commonly tested using contact angle measurements, where a high contact angle indicates hydrophobicity and a low angle signifies hydrophilicity. Techniques such as sessile drop, captive bubble, and Wilhelmy plate methods provide quantitative data on wettability and surface energy. Advanced instruments like goniometers and tensiometers enable precise analysis of surface interactions critical for material characterization and quality control.
Future Trends and Innovations in Material Science
Advancements in material science are driving the development of hybrid surfaces that combine hydrophobic and hydrophilic properties to create smart, responsive materials with applications in self-cleaning fabrics, anti-fog coatings, and biomedical devices. Nanotechnology enables precise surface engineering at the molecular level, enhancing water repellency or absorption based on environmental triggers, improving efficiency in water harvesting and filtration systems. Emerging research focuses on sustainable, bio-inspired materials that mimic natural hydrophobic and hydrophilic interactions, promoting eco-friendly innovations with enhanced durability and multifunctionality.
Hydrophobic Infographic
 libterm.com
libterm.com