Chemical processes play a crucial role in manufacturing, pharmaceuticals, and environmental management, influencing the quality and safety of products. Understanding chemical reactions and compounds enables you to optimize industrial applications and innovate sustainable solutions. Explore the rest of this article to uncover key insights about chemical principles and their real-world applications.
Table of Comparison
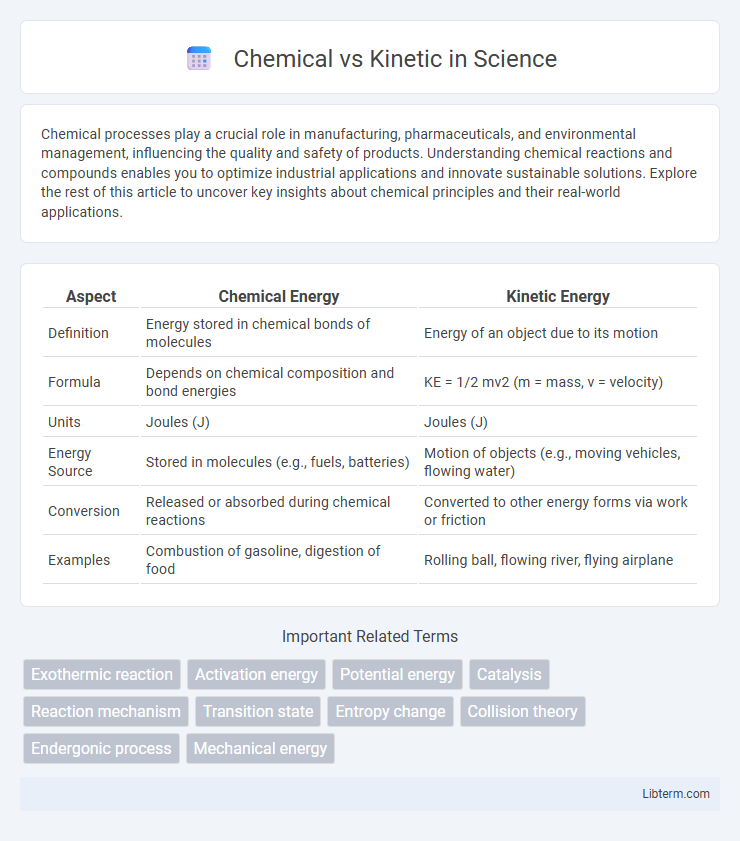
| Aspect | Chemical Energy | Kinetic Energy |
|---|---|---|
| Definition | Energy stored in chemical bonds of molecules | Energy of an object due to its motion |
| Formula | Depends on chemical composition and bond energies | KE = 1/2 mv2 (m = mass, v = velocity) |
| Units | Joules (J) | Joules (J) |
| Energy Source | Stored in molecules (e.g., fuels, batteries) | Motion of objects (e.g., moving vehicles, flowing water) |
| Conversion | Released or absorbed during chemical reactions | Converted to other energy forms via work or friction |
| Examples | Combustion of gasoline, digestion of food | Rolling ball, flowing river, flying airplane |
Introduction to Chemical and Kinetic Energy
Chemical energy is stored within the bonds of molecules and released during chemical reactions, playing a crucial role in processes such as combustion and metabolism. Kinetic energy refers to the energy possessed by an object due to its motion, quantified by the mass and velocity of the object. Both forms of energy are fundamental in physics and chemistry, with chemical energy often transforming into kinetic energy during energy transfer.
Defining Chemical Energy
Chemical energy is the potential energy stored within the bonds of atoms and molecules, released or absorbed during chemical reactions. This form of energy differs fundamentally from kinetic energy, which is the energy possessed by an object due to its motion. Understanding chemical energy involves examining the molecular structure changes that result in energy transfer, crucial for processes like combustion and metabolism.
Defining Kinetic Energy
Kinetic energy is defined as the energy possessed by an object due to its motion, mathematically expressed as half the product of the object's mass and the square of its velocity (KE = 1/2 mv2). Unlike chemical energy, which is stored in the bonds of molecules and released during chemical reactions, kinetic energy is directly related to the movement of particles or bodies. Understanding kinetic energy is fundamental in physics as it explains motion dynamics, collision outcomes, and energy transfer mechanisms.
Key Differences Between Chemical and Kinetic Energy
Chemical energy is stored within the bonds of atoms and molecules, released during chemical reactions, while kinetic energy pertains to the motion of objects and particles. The magnitude of chemical energy depends on the types of chemical bonds and molecular arrangements, contrasting with kinetic energy, which varies with an object's mass and velocity. Chemical energy transforms into kinetic energy during processes such as combustion, exemplifying their interrelated yet distinct nature.
Real-World Examples of Chemical Energy
Chemical energy is stored in the bonds of molecules and is released during chemical reactions, such as the combustion of gasoline in car engines or the metabolic processes in the human body that convert food into usable energy. In contrast, kinetic energy is associated with the motion of objects, exemplified by a moving vehicle or flowing water in a hydroelectric dam. Real-world examples of chemical energy include batteries powering electronic devices and the energy produced during the digestion of food, highlighting its vital role in both technological and biological systems.
Real-World Examples of Kinetic Energy
Kinetic energy is visible in everyday phenomena such as moving vehicles, flowing rivers, and winds driving wind turbines, showcasing its fundamental role in mechanical systems. Chemical energy stored in fuels is converted into kinetic energy when cars accelerate or rockets launch, highlighting the transformation from stored energy to motion. Windmills harness kinetic energy from air currents, directly converting natural motion into usable power without chemical intermediaries.
Energy Transformation: Chemical to Kinetic
Chemical energy stored in substances like fuels or food converts into kinetic energy during processes such as combustion or metabolism. This transformation occurs when chemical bonds break, releasing energy that propels objects or organisms into motion. Examples include engines converting gasoline's chemical energy into mechanical movement and muscles transforming stored nutrients into physical activity.
Applications in Everyday Life
Kinetic energy powers everyday activities such as walking, driving, and operating machines, converting motion into useful work. Chemical energy, stored in substances like batteries and fuel, enables devices to run and fuels transportation by releasing energy during reactions. Both energy types are essential for powering modern technology, from smartphones to vehicles and household appliances.
Pros and Cons of Chemical vs Kinetic Energy
Chemical energy stores potential in molecular bonds, releasing energy during chemical reactions, enabling efficient energy storage and portability but posing challenges such as limited recharge cycles and environmental hazards from fuel combustion. Kinetic energy, manifested through motion, offers immediate usability and high energy transfer efficiency without chemical waste, yet depends on continuous motion and cannot be stored directly, limiting its application for long-term energy storage. Balancing these attributes is crucial in selecting appropriate energy forms for applications ranging from transportation to industrial processes.
Conclusion: Choosing the Right Energy Source
Chemical energy offers high energy density and easy storage, making it ideal for portable applications and long-duration use; kinetic energy, being directly harnessed from motion, provides clean, renewable power suited for dynamic environments. The choice depends on specific needs such as energy efficiency, environmental impact, and application context, with chemical energy favored for high-output requirements and kinetic energy preferred for sustainability and reduced emissions. Evaluating factors like energy conversion efficiency and resource availability ensures selecting the most effective and practical energy source.
Chemical Infographic
 libterm.com
libterm.com