Hypertonic solutions contain a higher concentration of solutes compared to the inside of a cell, causing water to move out of the cell and potentially leading to cell shrinkage. These solutions are commonly used in medical treatments to reduce swelling or remove excess fluid from tissues. Discover how hypertonic solutions work and their important applications in healthcare throughout the rest of this article.
Table of Comparison
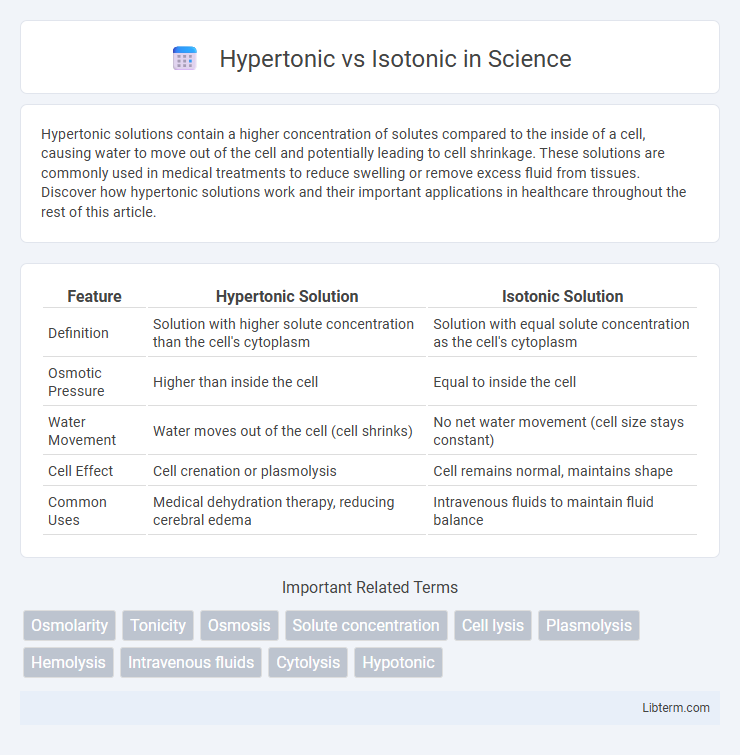
| Feature | Hypertonic Solution | Isotonic Solution |
|---|---|---|
| Definition | Solution with higher solute concentration than the cell's cytoplasm | Solution with equal solute concentration as the cell's cytoplasm |
| Osmotic Pressure | Higher than inside the cell | Equal to inside the cell |
| Water Movement | Water moves out of the cell (cell shrinks) | No net water movement (cell size stays constant) |
| Cell Effect | Cell crenation or plasmolysis | Cell remains normal, maintains shape |
| Common Uses | Medical dehydration therapy, reducing cerebral edema | Intravenous fluids to maintain fluid balance |
Introduction to Hypertonic and Isotonic Solutions
Hypertonic solutions contain a higher concentration of solutes compared to the inside of a cell, causing water to move out of the cell, leading to cell shrinkage. Isotonic solutions have equal solute concentrations inside and outside the cell, allowing water to move freely without changing cell size. These properties are critical in medical applications such as intravenous fluid therapy to maintain cellular balance.
Key Differences Between Hypertonic and Isotonic Solutions
Hypertonic solutions have a higher concentration of solutes compared to isotonic solutions, leading to water movement out of cells and causing them to shrink. Isotonic solutions maintain equal solute concentration inside and outside the cell, preventing net water movement and preserving cell size. These differences are critical in medical treatments such as intravenous therapy, where hypertonic solutions can reduce cellular edema, while isotonic solutions maintain fluid balance without altering cell volume.
Chemical Composition and Osmolarity Explained
Hypertonic solutions contain a higher concentration of solutes, such as sodium chloride or glucose, compared to the intracellular fluid, resulting in an osmolarity greater than 300 mOsm/L that draws water out of cells. Isotonic solutions have a chemical composition with solute concentrations roughly equal to that of intracellular fluid, maintaining an osmolarity close to 280-300 mOsm/L, which prevents net water movement across cell membranes. These differences in osmolarity and solute concentration are critical for cellular hydration and fluid balance in medical treatments like IV therapy.
Effects on Cells: Hypertonic vs Isotonic
Hypertonic solutions cause cells to shrink as water moves out due to higher solute concentration outside the cell, leading to crenation in animal cells and plasmolysis in plant cells. Isotonic solutions maintain cell size and shape by providing an equal concentration of solutes inside and outside the cell, allowing water to move freely without net gain or loss. This balance prevents cellular damage and supports normal physiological function.
Medical Applications of Hypertonic Solutions
Hypertonic solutions are widely used in medical applications to reduce cerebral edema by drawing water out of swollen brain cells, thereby decreasing intracranial pressure. They are essential in managing hyponatremia by raising serum sodium levels safely and effectively. Hypertonic saline also improves hemodynamics in patients with hypovolemic shock by expanding plasma volume more rapidly than isotonic solutions.
Uses of Isotonic Solutions in Healthcare
Isotonic solutions, such as 0.9% sodium chloride (normal saline) and lactated Ringer's solution, are widely used in healthcare to restore fluid balance without altering cell volume. They are essential in treating dehydration, blood loss, and maintaining intravenous access during surgery or medical procedures. These solutions help maintain hemodynamic stability and electrolyte balance, making them critical in emergency medicine and postoperative care.
Risks and Side Effects: Hypertonic vs Isotonic
Hypertonic solutions carry risks such as cellular dehydration, vein irritation, and potential hypernatremia due to their high solute concentration, which can lead to fluid shifts and electrolyte imbalances. Isotonic solutions generally have fewer side effects, as they maintain fluid balance without causing significant shifts in osmolality, but can still cause fluid overload in susceptible patients like those with heart or kidney conditions. Careful monitoring is essential with hypertonic fluids to avoid complications like central pontine myelinolysis, whereas isotonic fluids require vigilance to prevent edema and electrolyte disturbances.
Choosing the Right Solution for Medical Treatments
Selecting the appropriate solution for medical treatments depends on the patient's cellular hydration status and specific clinical needs. Hypertonic solutions draw water out of cells, effectively reducing edema and increasing extracellular fluid volume, making them ideal for conditions like cerebral edema or hyponatremia. Isotonic solutions maintain fluid balance without altering cell volume, commonly used for fluid replacement in dehydration or blood loss to stabilize blood pressure and electrolyte levels.
Common Examples of Hypertonic and Isotonic Solutions
Common examples of hypertonic solutions include 3% saline and 5% dextrose in normal saline, frequently used to reduce cerebral edema and treat hyponatremia. Isotonic solutions such as 0.9% normal saline and lactated Ringer's solution are widely administered for fluid resuscitation and maintaining hydration without causing fluid shifts across cell membranes. Selecting hypertonic or isotonic solutions depends on the clinical objective of modifying intracellular volume or restoring extracellular fluid balance.
Summary: Selecting Hypertonic or Isotonic for Optimal Results
Choosing between hypertonic and isotonic solutions depends on the desired cellular response and hydration goals. Hypertonic solutions cause cells to shrink by drawing water out, effective for reducing swelling or promoting dehydration, while isotonic solutions maintain cell size by balancing fluid exchange, ideal for hydration and restoring electrolyte balance. Evaluating patient needs and treatment objectives ensures optimal results through appropriate solution selection.
Hypertonic Infographic
 libterm.com
libterm.com