Catalysis accelerates chemical reactions by lowering activation energy, enabling faster product formation without being consumed in the process. Autocatalysis occurs when the reaction product itself acts as a catalyst, creating a feedback loop that intensifies the reaction rate. Discover how these powerful mechanisms influence chemical processes and explore their practical applications in the rest of the article.
Table of Comparison
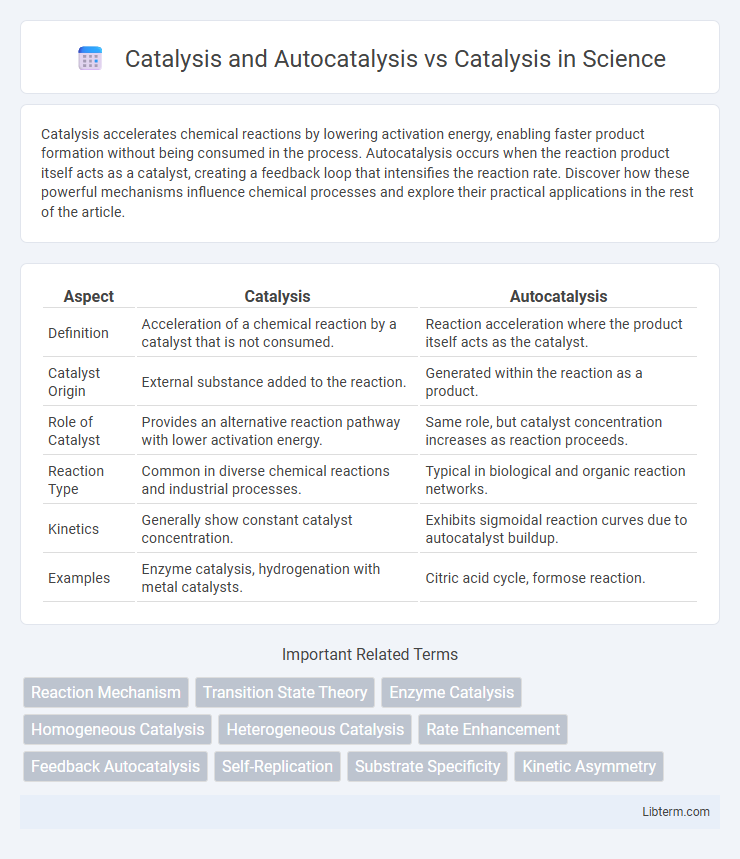
| Aspect | Catalysis | Autocatalysis |
|---|---|---|
| Definition | Acceleration of a chemical reaction by a catalyst that is not consumed. | Reaction acceleration where the product itself acts as the catalyst. |
| Catalyst Origin | External substance added to the reaction. | Generated within the reaction as a product. |
| Role of Catalyst | Provides an alternative reaction pathway with lower activation energy. | Same role, but catalyst concentration increases as reaction proceeds. |
| Reaction Type | Common in diverse chemical reactions and industrial processes. | Typical in biological and organic reaction networks. |
| Kinetics | Generally show constant catalyst concentration. | Exhibits sigmoidal reaction curves due to autocatalyst buildup. |
| Examples | Enzyme catalysis, hydrogenation with metal catalysts. | Citric acid cycle, formose reaction. |
Introduction to Catalysis
Catalysis involves accelerating chemical reactions by lowering the activation energy through a catalyst, which remains unchanged after the reaction. Autocatalysis is a specific type where the reaction product itself acts as the catalyst, enhancing the process as it proceeds. Understanding these mechanisms is crucial in industrial processes and biochemical systems to optimize reaction rates and efficiencies.
Fundamentals of Autocatalysis
Autocatalysis is a unique reaction mechanism where the product itself acts as a catalyst, accelerating its own formation and creating a positive feedback loop absent in general catalysis. This fundamental difference results in nonlinear kinetics characterized by sigmoidal concentration-time profiles and potential reaction rate amplification. Understanding these dynamics is crucial for applications in chemical synthesis, biological systems, and materials science where autocatalytic behavior influences reaction efficiency and system stability.
Differences Between Catalysis and Autocatalysis
Catalysis involves a catalyst that accelerates a chemical reaction without being consumed, while autocatalysis occurs when one of the reaction products acts as the catalyst for the same reaction. In catalysis, an external substance facilitates the reaction, whereas in autocatalysis, the process is self-sustaining due to the product's catalytic role. This fundamental difference means autocatalysis often leads to nonlinear kinetics, contrasting the typically steady rate enhancement seen in ordinary catalysis.
Mechanisms of Catalytic Reactions
Catalysis involves the acceleration of chemical reactions through the presence of a catalyst that lowers the activation energy by providing an alternative reaction pathway, often via adsorption and intermediate complex formation. Autocatalysis differs as the reaction product itself functions as the catalyst, creating a self-amplifying mechanism where reaction rates increase with product concentration, frequently observed in nonlinear kinetics. Mechanistically, catalytic reactions proceed through steps including substrate binding, transformation, and product release, whereas autocatalytic cycles emphasize feedback loops enhancing catalytic sites without external catalysts.
Types of Catalysts and Their Roles
Catalysis involves the acceleration of chemical reactions by catalysts, which can be classified into heterogeneous, homogeneous, and biocatalysts based on their phase and origin. Autocatalysis is a unique form where the reaction product itself acts as a catalyst, enhancing the reaction rate in a self-sustaining manner. Heterogeneous catalysts provide surface sites for reactant adsorption, homogeneous catalysts operate within the same phase as reactants for molecular-level interaction, and biocatalysts such as enzymes offer specificity and efficiency, while autocatalysts propagate reaction pathways through product-catalyzed loops.
Autocatalytic Reaction Pathways
Autocatalytic reaction pathways uniquely accelerate chemical processes by generating catalysts as products, thereby enhancing reaction rates through self-amplification mechanisms. Unlike traditional catalysis, which relies on external catalysts to lower activation energy without being consumed, autocatalysis intertwines catalyst formation and consumption within the same reaction cycle. This dynamic behavior is critical in fields like biochemistry and materials science, where autocatalytic networks drive complex system evolutions and emergent properties.
Industrial Applications of Catalysis
Catalysis enhances industrial chemical processes by increasing reaction rates and selectivity, crucial in petrochemical refining, pharmaceuticals, and polymer production. Autocatalysis, where a product catalyzes its own formation, is less common in industrial applications due to complexity in control and scalability. Industrial catalysis prioritizes heterogeneous and homogeneous catalysts for efficiency, durability, and ease of separation, driving innovations in catalysts such as zeolites, metal complexes, and enzymes for sustainable manufacturing.
Significance of Autocatalysis in Chemistry
Autocatalysis plays a significant role in chemical reactions by enabling a product to act as a catalyst for its own formation, thereby accelerating reaction rates and creating feedback mechanisms that are essential in biochemical processes such as enzyme activity and metabolic pathways. Unlike general catalysis, where an external catalyst lowers activation energy, autocatalysis drives self-propagating reactions that are critical in systems chemistry, origin-of-life studies, and synthetic reaction networks. The distinctive feature of autocatalysis lies in its capacity to amplify reaction outcomes, making it fundamental for understanding exponential growth phenomena and complex chemical behavior.
Comparative Advantages: Catalysis vs Autocatalysis
Catalysis enhances reaction rates by using external substances, while autocatalysis involves a product of the reaction accelerating the process, providing self-sustainability and efficiency in reaction progression. Autocatalysis offers advantages in reaction control and feedback mechanisms, making it valuable in biochemical and industrial processes where continuous activation is crucial. Catalysis generally provides broader applicability and easier modulation through catalyst selection, favoring diverse chemical synthesis with precise rate control.
Future Perspectives in Catalytic Science
Future perspectives in catalytic science emphasize the integration of autocatalysis to enhance reaction efficiency and selectivity by leveraging self-sustaining catalytic cycles. Advances in nanomaterials and enzyme-inspired catalysts are expected to drive innovations in sustainable chemical processes, lowering energy consumption and enabling carbon-neutral synthesis. The exploration of autocatalytic networks offers promising opportunities for developing adaptive catalytic systems with applications in green chemistry and artificial metabolism.
Catalysis and Autocatalysis Infographic
 libterm.com
libterm.com