Covalent provides a powerful API that simplifies access to blockchain data by aggregating information from multiple sources into a single, unified endpoint. This ease of integration enables developers to build decentralized applications, analytics, and tools efficiently while ensuring data accuracy and completeness. Discover how Covalent can transform Your blockchain projects by reading the rest of this article.
Table of Comparison
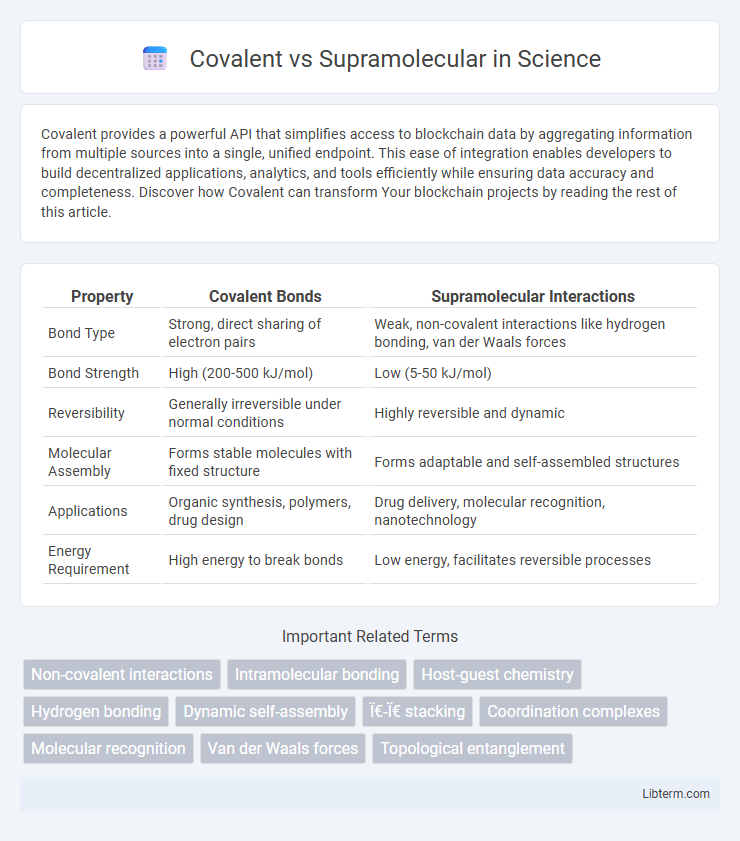
| Property | Covalent Bonds | Supramolecular Interactions |
|---|---|---|
| Bond Type | Strong, direct sharing of electron pairs | Weak, non-covalent interactions like hydrogen bonding, van der Waals forces |
| Bond Strength | High (200-500 kJ/mol) | Low (5-50 kJ/mol) |
| Reversibility | Generally irreversible under normal conditions | Highly reversible and dynamic |
| Molecular Assembly | Forms stable molecules with fixed structure | Forms adaptable and self-assembled structures |
| Applications | Organic synthesis, polymers, drug design | Drug delivery, molecular recognition, nanotechnology |
| Energy Requirement | High energy to break bonds | Low energy, facilitates reversible processes |
Introduction to Covalent and Supramolecular Chemistry
Covalent chemistry involves the formation of strong chemical bonds through the sharing of electron pairs between atoms, resulting in stable molecules with well-defined structures. Supramolecular chemistry studies the non-covalent interactions such as hydrogen bonding, van der Waals forces, and metal coordination that drive the assembly of discrete molecular entities into larger, complex architectures. Understanding the principles of covalent bonding and supramolecular interactions is essential for designing advanced materials, molecular machines, and targeted drug delivery systems.
Defining Covalent Bonds
Covalent bonds involve the sharing of electron pairs between atoms, creating strong, stable connections that form the backbone of molecular structures. These bonds result in fixed, well-defined molecular geometries due to directional electron sharing. Supramolecular interactions, in contrast, consist of weaker, non-covalent forces such as hydrogen bonding, van der Waals forces, and p-p stacking, which enable reversible and dynamic assemblies without altering the covalent framework.
Understanding Supramolecular Interactions
Supramolecular interactions involve non-covalent forces such as hydrogen bonding, van der Waals forces, and p-p stacking, which enable reversible and dynamic assembly of molecular components. Unlike covalent bonds that form strong, permanent connections by electron sharing, supramolecular chemistry focuses on molecular recognition and self-assembly processes governed by weak, highly specific interactions. These interactions are critical in fields like drug delivery, molecular sensors, and nanotechnology due to their adaptability and self-healing properties.
Structural Differences and Hierarchies
Covalent structures consist of atoms bonded through strong, directional covalent bonds forming well-defined, stable molecular frameworks with fixed geometries and high bond energies typically above 150 kJ/mol. Supramolecular structures arise from non-covalent interactions such as hydrogen bonding, van der Waals forces, and p-p stacking, resulting in dynamic, reversible assemblies with hierarchical organization ranging from molecular to macroscopic scales. These structural hierarchies in supramolecular systems enable adaptive functions and self-healing properties absent in rigid covalent networks, highlighting distinct organization principles and energy scales governing their stability and assembly.
Bond Strength: Covalent vs. Supramolecular
Covalent bonds exhibit high bond strength, typically ranging from 150 to 1100 kJ/mol, due to the sharing of electron pairs between atoms, resulting in stable and durable molecular structures. Supramolecular interactions, such as hydrogen bonding, van der Waals forces, and p-p stacking, have significantly lower bond energies, usually between 5 and 50 kJ/mol, enabling reversible and dynamic assembly processes. The substantial difference in bond strength influences material properties, with covalent bonds providing rigidity and permanence, while supramolecular bonds offer adaptability and self-healing capabilities.
Formation Mechanisms
Covalent bonds form through the sharing of electron pairs between atoms, creating strong, stable connections essential in chemical compounds and molecular frameworks. Supramolecular assemblies arise from non-covalent interactions such as hydrogen bonding, van der Waals forces, and electrostatic interactions, allowing reversible and dynamic self-organization. These distinct formation mechanisms dictate the stability, functionality, and adaptability of covalent versus supramolecular systems in materials science and biological processes.
Functional Applications in Modern Science
Covalent and supramolecular chemistries offer distinct functional advantages in modern science, with covalent bonds providing robust, stable frameworks essential for drug design, polymer synthesis, and materials engineering. Supramolecular interactions enable reversible, adaptive assemblies crucial for molecular recognition, targeted drug delivery, and responsive smart materials. These complementary approaches drive innovation in nanotechnology, catalysis, and biomolecular engineering by leveraging permanent covalent linkages alongside dynamic, non-covalent supramolecular systems.
Stability and Reversibility
Covalent bonds exhibit high stability due to strong electron sharing between atoms, resulting in robust and permanent molecular structures. Supramolecular interactions rely on weaker non-covalent forces such as hydrogen bonding, van der Waals forces, and ionic interactions, which provide greater reversibility and dynamic assembly. The reversible nature of supramolecular systems enables stimuli-responsive behavior and self-healing properties that covalent bonds typically lack.
Material Properties Comparison
Covalent materials exhibit strong, directional bonds resulting in high mechanical strength, thermal stability, and chemical resistance. Supramolecular materials rely on weaker, reversible non-covalent interactions such as hydrogen bonding, metal coordination, and van der Waals forces, enabling self-healing, stimuli-responsiveness, and easier processability. The dynamic nature of supramolecular assemblies offers tunable material properties, but covalent networks generally provide superior durability and rigidity for structural applications.
Future Perspectives and Emerging Trends
Future perspectives in covalent chemistry emphasize the development of dynamic covalent bonds enabling adaptive and self-healing materials with enhanced stability and functionality. Emerging trends in supramolecular chemistry focus on harnessing non-covalent interactions for programmable molecular assemblies, leading to innovations in responsive drug delivery systems and nanoscale machines. Integration of covalent and supramolecular approaches promises breakthroughs in multifunctional materials and smart, stimuli-responsive technologies.
Covalent Infographic
 libterm.com
libterm.com